Top Qs
Timeline
Chat
Perspective
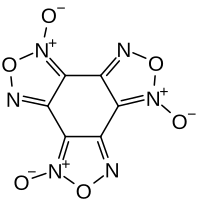
Benzotrifuroxan
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Benzotrifuroxan is a heterocyclic organic compound that is related to 1,2,5-oxadioles. The high-energy compound is explosive.
Remove ads
History
The compound was first synthesized in 1924 by O. Turek as hexanitrosobenzene.[1][2] In addition to the hexanitroso structure, symmetric polycyclic structures could also be formulated.[3][4]
Characteristics
Physical properties
Benzotrifuroxan is a crystalline solid that melts at 195 °C.[5] The compound crystallizes in an orthorhombic crystal lattice with the space group Pna21.[4][6] The molar enthalpy of formation is 606 kJ·mol−1, the enthalpy of combustion is −2967 kJ·mol−1.[7]
Chemical properties
Benzotrifuroxan can decompose explosively. The heat of explosion is 5903 kJ·kg −1,[8] the detonation speed is 8.61 km·s −1.[9] The compound is sensitive to impact.[10]
Benzotrifuroxan forms stable complexes with aromatic hydrocarbons such as naphthalene, 1-phenylnaphthalene, 2-phenylnaphthalene and tetrahydronaphthalene. Recrystallization in benzene yields a 1:1 complex with the solvent, whereby the benzene can only be removed at 100 °C in vacuum.[11]
Remove ads
Synthesis
Benzotrifuroxan can be obtained by thermal degradation of 1,3,5-triazido-2,4,6-trinitrobenzene.[1][2]
A further synthesis can be carried out by reacting 5,7-dichloro-4,6-dinitronbenzofuroxan with sodium azide.[12]
Uses
In combination with TNT, the compound can be used to produce nanodiamonds using detonation shock waves.[13]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads