Top Qs
Timeline
Chat
Perspective
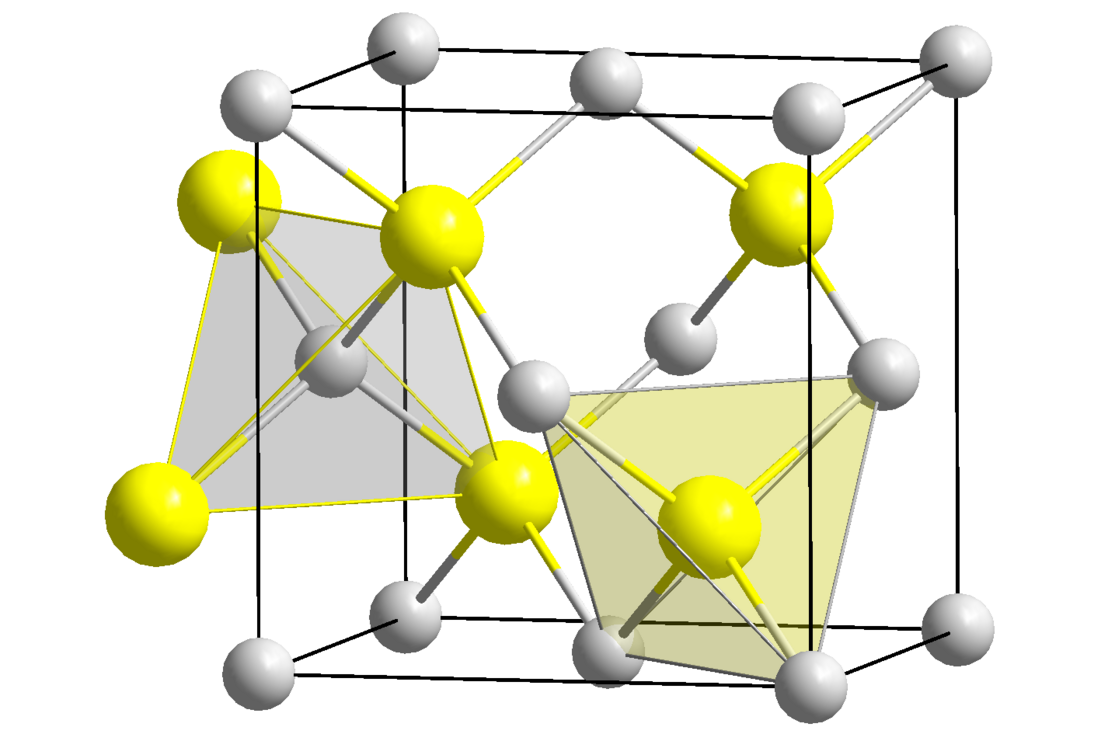
Beryllium sulfide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Beryllium sulfide (BeS) is an ionic compound from the sulfide group with the formula BeS. It is a white solid with a sphalerite structure that is decomposed by water and acids.[3]
Remove ads
Preparation
Beryllium sulfide powders can be prepared by the reaction of sulfur and beryllium in a hydrogen atmosphere by heating the mixture for 10-20 minutes at temperatures from 1000-1300 °C. If done at 900 °C, there is beryllium metal impurities.[4]
Alternatively, it can be prepared by the reaction of beryllium chloride and hydrogen sulfide at 900 °C.[3][4]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads