Top Qs
Timeline
Chat
Perspective
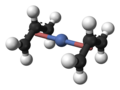
Bis(allyl)nickel
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Bis(allyl)nickel is an organonickel compound with the formula Ni(η3-C3H5)2. The molecule consists of two allyl ligands bound to nickel(II). It has inversion symmetry.[1] It is a volatile yellow liquid at room temperature.
Remove ads
Preparation and reactions
It can be prepared by the reaction of allyl magnesium bromide with anhydrous nickel chloride.[2] It was first prepared similarly by Gunther Wilke et al. The same group reported that the complex react with carbon monoxide to give nickel tetracarbonyl and 1,5-hexadiene. It catalyzes the trimerization of butadiene.[3] With tertiary phosphines, the complex gives the tetrakis derivative. Such reactions to proceed via the intermediacy of the 18-electron adduct.[4]
- Ni(C3H5)2 + PR3 → Ni(C3H5)2(PR3)
- Ni(C3H5)2PR3 → Ni(CH2=CHCH2CH2CH=CH2)(PR3)
- Ni(CH2=CHCH2CH2CH=CH2)(PR3) + 3 PR3 → Ni(PR3)4 + CH2=CHCH2CH2CH=CH2
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads