Top Qs
Timeline
Chat
Perspective
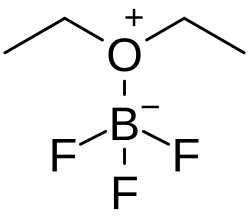
Boron trifluoride etherate
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Boron trifluoride etherate, strictly boron trifluoride diethyl etherate, or boron trifluoride–ether complex, is the chemical compound with the formula BF3O(C2H5)2, often abbreviated BF3OEt2. It is a colorless liquid, although older samples can appear brown. The compound is used as a source of boron trifluoride in many chemical reactions that require a Lewis acid.[1] The compound features tetrahedral boron coordinated to a diethylether ligand.[2] Many analogues are known, including the methanol complex.
Remove ads
Reactions
Boron trifluoride etherate serves as a source of boron trifluoride according to the equilibrium:
- BF3OEt2 BF3 + OEt2
The BF3 binds to even weak Lewis bases, inducing reactions of the resulting adducts with nucleophiles.[1]
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads