Top Qs
Timeline
Chat
Perspective
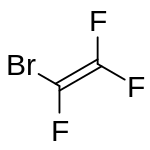
Bromotrifluoroethylene
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Bromotrifluoroethylene (BTFE) is a halogenated ethylene derivative with the chemical formula F2CCBrF. It is a highly flammable colourless gas with a musty odour resembling phosgene. It can polymerise spontaneously.[3]
Remove ads
Preparation
Bromotrifluoroethylene can be prepared from chlorotrifluoroethylene with high yields:[3][4]
- F2C=ClF + HBr → CF2BrCHClF + Zn → CF2=CHF + ZnBrCl
- CF2=CHF + Br2 → CF2BrCHBrF + KOH → CF2=CFBr + KBr + H2O
It was first prepared by the Belgian chemist Frédéric Swarts in 1899.[5]
Reactions and uses
Bromotrifluoroethylene forms metal complexes with substituted phosphine compounds and platinum(II).[6]
BTFE can polymerise on standing. Spontaneous polymerisation may be inhibited by addition of tributylamine.[4] UV light and heat may accelerate polymerisation.[4] It participates in various co-polymerisation reactions.[2] BTFE telomers are oily liquids sold under the tradename BFC oil. The telomers can be prepared with fluorotrichloromethane or tetrachloromethane as telogens. If tetrachloromethane is used for the telomerisation, it will have -CCl3 terminals.[5] It is a useful reagent for the synthesis of trifluorovinyl compounds.[3]
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads