Top Qs
Timeline
Chat
Perspective
Cadmium hydroxide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Cadmium hydroxide is an inorganic compound with the formula Cd(OH)2. It is a white crystalline ionic compound that is a key component of nickel–cadmium battery.[5]
Remove ads
Structure
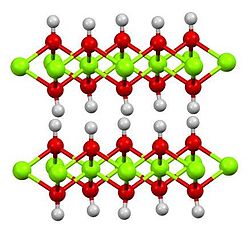
Cadmium hydroxide adopts the same structure as Mg(OH)2, consisting of slabs of metal centers, each bonded by six hydroxide ligands.[6] The Cd(OH)2 structure is a recurring motif in inorganic chemistry. For example it is adopted by vanadium ditelluride.[7]
Preparation, and reactions
Cadmium hydroxide is produced by treating an aqueous solution containing Cd2+ (say cadmium nitrate) with sodium hydroxide:[8][5]
- Cd(NO3)2 + 2 NaOH → Cd(OH)2 + 2 NaNO3
Cd(OH)2 and cadmium oxide exhibit similar reactions. Cadmium hydroxide is more basic than zinc hydroxide. It forms the anionic complex [Cd(OH)4]2− when treated with concentrated base. It forms complexes with cyanide, thiocyanate, and ammonia.
Cadmium hydroxide loses water on heating, producing cadmium oxide. Decomposition commences at 130 °C and is complete at 300 °C. Reactions with mineral acids (HX) gives the corresponding cadmium salts (CdX2). With hydrochloric acid, sulfuric acid, and nitric acid, the products are cadmium chloride, cadmium sulfate, and cadmium nitrate, respectively.[8][5]
Remove ads
Uses
It is generated in storage battery anodes, in nickel-cadmium and silver-cadmium storage batteries in its discharge:
- 2 NiO(OH) + 2 H2O + Cd → Cd(OH)2 + 2 Ni(OH)2
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads