Top Qs
Timeline
Chat
Perspective
Caesium bromide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
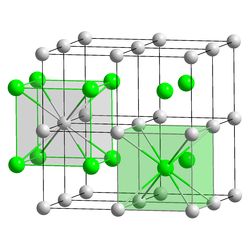
Caesium bromide or cesium bromide is an ionic compound of caesium and bromine with the chemical formula CsBr. It is a white or transparent solid with melting point at 636 °C that readily dissolves in water. Its bulk crystals have the cubic CsCl structure, but the structure changes to the rocksalt type in nanometer-thin film grown on mica, LiF, KBr or NaCl substrates.[6]
Remove ads
Synthesis
Caesium bromide can be prepared via following reactions:
- CsOH (aq) + HBr (aq) → CsBr (aq) + H2O (l)
- Cs2(CO3) (aq) + 2 HBr (aq) → 2 CsBr (aq) + H2O (l) + CO2 (g)
- Direct synthesis:
- 2 Cs (s) + Br2 (g) → 2 CsBr (s)
The direct synthesis is a vigorous reaction of caesium with bromine. Due to its high cost, it is not used for preparation.
Uses
Caesium bromide is sometimes used in optics as a beamsplitter component in wide-band spectrophotometers.
References
Cited sources
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads