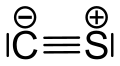Top Qs
Timeline
Chat
Perspective
Carbon monosulfide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Carbon monosulfide is a chemical compound with the formula CS. This diatomic molecule is the sulfur analogue of carbon monoxide, and is unstable as a solid or a liquid, but it has been observed as a gas both in the laboratory and in the interstellar medium.[1] The molecule resembles carbon monoxide with a triple bond between carbon and sulfur. The molecule is not intrinsically unstable, but it tends to polymerize in sunlight to a brown mass, as first discovered in 1868 and 1872.[2] The polymer is quite stable, decomposing a little at 360 °C to carbon disulfide. This tendency towards polymerization reflects the greater stability of C–S single bonds.
Polymers with the formula (CS)n have been reported,[3] and the formal dimer is ethenedithione. Also, CS has been observed as a ligand in some transition metal complexes.[citation needed]
The simplest carbon monosulfide synthesis decomposes carbon disulfide in a high-voltage AC arc.[4]
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads