Top Qs
Timeline
Chat
Perspective
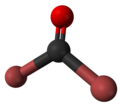
Carbonyl bromide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Carbonyl bromide, also known as bromophosgene, is a carbon oxohalide and a bromine analogue of phosgene, with the chemical formula COBr2. It is a colorless liquid. Carbonyl bromide is a decomposition product of halon compounds used in fire extinguishers.[2]
Remove ads
Synthesis and reactions
Carbonyl bromide is formed by the oxidation of carbon tetrabromide with sulfuric acid:
- CBr4 + H2SO4 → COBr2 + SO2 + Br2 + H2O
In contrast to phosgene, carbonyl bromide cannot be produced efficiently by halogenation of carbon monoxide. The bromination of carbon monoxide follows this equation:
- CO + Br2 ⇌ COBr2
But the process is slow at room temperature. Increasing temperature, in order to increase the reaction rate, results in a further shift of the chemical equilibrium towards the educts (since ΔRH < 0 and ΔRS < 0).[3][4][clarification needed]
Carbonyl bromide slowly decomposes to carbon monoxide and elemental bromine even at low temperatures.[5] It is also sensitive to hydrolysis, breaking down into hydrogen bromide and carbon dioxide.
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads