Top Qs
Timeline
Chat
Perspective
Chromium trioxide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Chromium trioxide (also known as chromium(VI) oxide or chromic anhydride) is the inorganic compound with the formula CrO3.[7] It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name.[6]
This compound is a dark-purple solid. Millions of kilograms are produced annually, mainly for electroplating.[8] Chromium trioxide is a powerful oxidiser, a mutagen, and a carcinogen.[9]
Remove ads
Production and structure
Chromium trioxide is generated by treating sodium dichromate with sulfuric acid:[6]
- H2SO4 + Na2Cr2O7 → 2 CrO3 + Na2SO4 + H2O
Approximately 100,000 tonnes are produced annually by this or similar routes.[8]
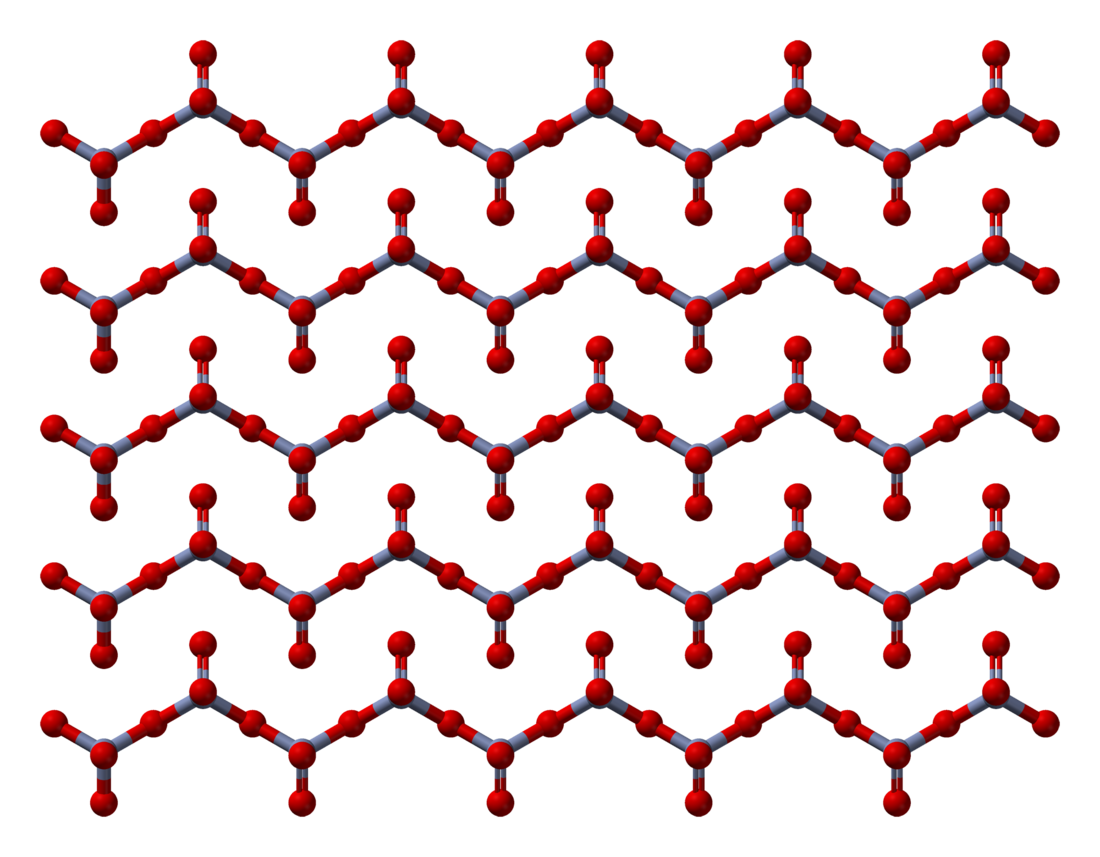
The solid consists of chains of tetrahedrally coordinated chromium atoms that share vertices. Each chromium center therefore shares two oxygen centers with neighbors. Two oxygen atoms are not shared, giving an overall stoichiometry of 1:3.[10][11]

The structure of monomeric CrO3 has been calculated using density functional theory, and is predicted to be pyramidal (point group C3v) rather than planar (point group D3h).[12]
Remove ads
Reactions
Chromium trioxide decomposes above 197 °C, liberating oxygen and eventually giving Cr2O3:[7]
- 4 CrO3 → 2 Cr2O3 + 3 O2
With water it converts to "chromic acid", which includes red-orange species such as H2CrO4 and H2Cr2O7.[7]
Chromium trioxide is a Lewis acid, forming adducts with many (non-oxidizable) bases, such as chloride:[13]
- CrO3 + Cl− → CrO3Cl−
Applications
Summarize
Perspective
Chromium trioxide is mainly used in chrome plating. It is typically employed with additives that affect the plating process but do not react with the trioxide. The trioxide reacts with cadmium, zinc, and other metals to generate passivating chromate films that resist corrosion. It is also used in the production of synthetic rubies. Chromic acid solution is also used in applying types of anodic coating to aluminium, which are primarily used in aerospace applications. On the International Space Station, it is used to control bacteria growth in the wastewater storage tank. A chromic acid/phosphoric acid solution is also the preferred stripping agent of anodic coatings of all types.
Organic chemistry
Chromium trioxide and a variety of its derivatives are used in organic chemistry. Some of these reagents:
- CrO3(pyridine)2.[14] When dissolved in pyridine, this complex is called Sarett's reagent.
- Collins reagent is a solution CrO3(pyridine)2 but in dichloromethane.[15]
- pyridinium chlorochromate (PCC) is the pyridinium salt of the chloride adduct of chromium trichloride, [CrO3Cl]−.
Typically these reagents convert alcohols to carbonyls:[16]
- 4 CrO3 + 3 RCH2OH + 12 H+ → 3 RCOOH + 4 Cr3+ + 9 H2O
- 2 CrO3 + 3 R2CHOH + 6 H+ → 3 R2C=O + 2 Cr3+ + 6 H2O
Safety
Chromium trioxide is highly toxic, corrosive, and carcinogenic.[17] It is the main example of hexavalent chromium, an environmental hazard.[18] The related chromium(III) derivatives are not particularly dangerous; thus, reductants are used to destroy chromium(VI) samples.
Chromium trioxide, being a powerful oxidizer, will ignite organic materials such as alcohols on contact.
Images
- A concentrated solution of potassium dichromate in water.
- Addition of sulfuric acid to the solution.
- Crystallization of chromium trioxide from the reaction.
- Reaction between chromium trioxide and ethanol
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads