Top Qs
Timeline
Chat
Perspective
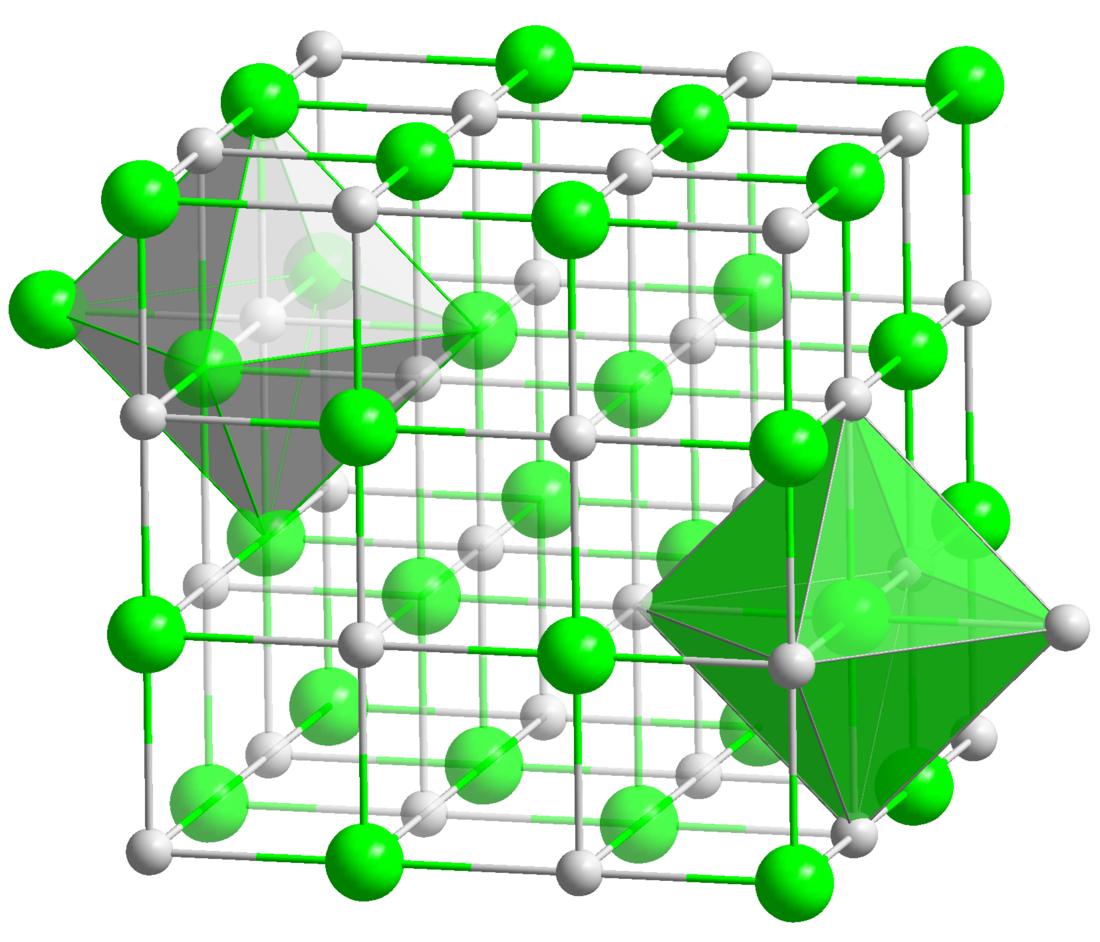
Chromium(II) oxide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Chromium(II) oxide (CrO) is an inorganic compound composed of chromium and oxygen.[1] It is a black powder that crystallises in the rock salt structure.[2] Hypophosphites may reduce chromium(III) oxide to chromium(II) oxide:
- H3PO2 + 2 Cr2O3 → 4 CrO + H3PO4
It is readily oxidized by the atmosphere. CrO is basic, while CrO3 is acidic, and Cr2O3 is amphoteric.[3]
CrO occurs in the spectra of luminous red novae, which occur when two stars collide. It is not known why red novae are the only objects that feature this molecule; one possible explanation is an as-yet-unknown nucleosynthesis process.[4]
Remove ads
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads