Top Qs
Timeline
Chat
Perspective
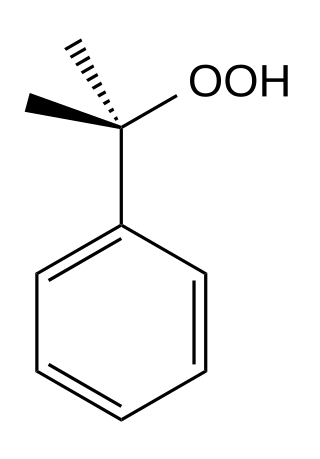
Cumene hydroperoxide
Aromatic organic chemical compound From Wikipedia, the free encyclopedia
Remove ads
Cumene hydroperoxide is the organic compound with the formula C6H5C(CH3)2OOH; this oily liquid is classified as an organic hydroperoxide.[2] Products of decomposition of cumene hydroperoxide are methylstyrene, acetophenone, and 2-phenylpropan-2-ol.[3]
It is produced by treatment of cumene with oxygen, an autoxidation. At temperatures >100 °C, oxygen is passed through liquid cumene:[4]
- C
6H
5CH(CH
3)
2 + O2 → C
6H
5C(CH
3)
2OOH
Dicumyl peroxide is a side product.
Remove ads
Applications
Cumene hydroperoxide is an intermediate in the cumene process for producing phenol and acetone from benzene and propene.
Cumene hydroperoxide is a radical initiator for production of acrylates.[5]
Cumene hydroperoxide is involved as an organic peroxide in the production of propylene oxide by the oxidation of propene. This technology was commercialized by Sumitomo Chemical.[6]
The oxidation by cumene hydroperoxide of propene affords propylene oxide and the byproduct 2-phenylpropan-2-ol. The reaction follows this stoichiometry:
- CH
3CHCH
2 + C
6H
5C(CH
3)
2OOH → CH
3CHCH
2O + C
6H
5C(CH
3)
2OH
Dehydrating and hydrogenating cumyl alcohol recycles the cumene.
Remove ads
Safety
Cumene hydroperoxide, like all organic peroxides, is potentially explosive. It is also toxic, corrosive and flammable as well as a skin-irritant.[7]
References
Related terms
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads