Top Qs
Timeline
Chat
Perspective
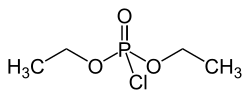
Diethyl phosphorochloridate
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Diethyl chlorophosphate is an organophosphorus compound with the formula (C2H5O)2P(O)Cl. As a reagent in organic synthesis, it is used to convert alcohols to the corresponding diethylphosphate esters. It is a colorless liquid with a fruity odor. It is a corrosive, and as a cholinesterase inhibitor, highly toxic through dermal absorption.[1] The molecule is tetrahedral.
Remove ads
Synthesis and reactions
The compound is prepared by the chlorination of diethylphosphite with carbon tetrachloride (Atherton–Todd reaction).[2]
The compound is electrophilic. Controlled hydrolysis gives tetraethyl pyrophosphate. Alcohols react to give phosphate esters: [3]
- (C2H5O)2P(O)Cl + ROH → (C2H5O)2P(O)OR + HCl
The reagent is routinely employed in organic synthesis for phosphorylation of carboxylates,[4] alcohols,[5] and amines.[6]
Remove ads
See also
- Diethyl chlorophosphate at www.chemicalbook.com.
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads