Top Qs
Timeline
Chat
Perspective
Dihalomethane
From Wikipedia, the free encyclopedia
Remove ads
The dihalomethanes are organic compounds in which two hydrogen atoms in methane are replaced by halogen atoms. They belong to the haloalkanes, specifically the subgroup of halomethanes, and contains ten members.
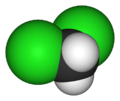
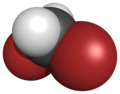
There are four members with only one kind of halogen atom: difluoromethane, dichloromethane, dibromomethane and diiodomethane.
| Structural Formula |  |
 |
 |
 |
| Name | Difluoromethane | Dichloromethane | Dibromomethane | Diiodomethane |
| Melting point | −136 °C[1] | −97 °C[2] | −52 °C[3] | 6 °C[4] |
| Boiling point | −51,7 °C[1] | 40 °C[2] | 97 °C[3] | decomposes[4] |
| Space-filling model |  |
 |
 |
 |
There are six members with two kinds of halogen atoms:
Remove ads
References
See also
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads
