Top Qs
Timeline
Chat
Perspective
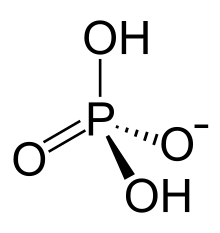
Dihydrogen phosphate
Inorganic ion From Wikipedia, the free encyclopedia
Remove ads
Dihydrogen phosphate is an inorganic ion with the formula [H2PO4]−. Phosphates occur widely in natural systems.[1] Perhaps the most common salt of dihydrogen phosphate is sodium dihydrogen phosphate. It is used in animal feed, fertilizer, buffer (in food), and treating metal surfaces.[2]
Remove ads
Structure
The dihydrogen phosphate anion consists of a central phosphorus atom bonded two oxides and two hydroxy groups in a tetrahedral arrangement.[3]
Acid-base equilibria
Dihydrogen phosphate can be both a hydrogen donor and acceptor.
Examples
- Ammonium dihydrogen phosphate ((NH4)(H2PO4))
- Monocalcium phosphate (Ca(H2PO4)2)
Safety
Many foods including milk, eggs, poultry, and nuts contain these sodium phosphates.[1]
Notes
- Values are at 25 °C and 0 ionic strength.
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads