Top Qs
Timeline
Chat
Perspective
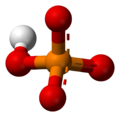
Monohydrogen phosphate
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Hydrogen phosphate or monohydrogen phosphate (systematic name) is the inorganic ion with the formula [HPO4]2-. Its formula can also be written as [PO3(OH)]2-. Together with dihydrogen phosphate, hydrogenphosphate occurs widely in natural systems. Their salts are used in fertilizers and in cooking.[1] Most hydrogenphosphate salts are colorless, water soluble, and nontoxic.
It is a conjugate acid of phosphate [PO4]3- and a conjugate base of dihydrogen phosphate [H2PO4]−.
It is formed when a pyrophosphate anion [P
2O
7]4−
reacts with water H
2O by hydrolysis, which can give hydrogenphosphate:
- [P
2O
7]4−
+ H2O ⇌ 2 [HPO
4]2−
Remove ads
Acid-base equilibria
Hydrogenphosphate is an intermediate in the multistep conversion of phosphoric acid to phosphate:
- Values are at 25 °C and 0 ionic strength.
Examples
- Diammonium phosphate, (NH4)2HPO4
- Disodium phosphate, Na2HPO4, with varying amounts of water of hydration
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads