Top Qs
Timeline
Chat
Perspective
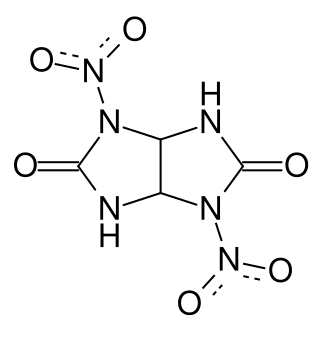
Dinitroglycoluril
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Dinitroglycoluril (DNGU) is a high explosive[1] chemical compound with the formula C4H4N6O6. Dinitroglycoluril is of growing interest due to its stability, ability to mix with oxygen positive explosives to form composites, and it is a precursor to tetranitroglycoluril.[2]
Remove ads
Preparation and decomposition
Dinitroglycoluril can be created by nitrating glycoluril with concentrated nitric acid.[3]
- C4H6N4O2 + 2 HNO3 → C4H4N6O6 + 2 H2O
The activation energy required to begin decomposition of dinitroglycoluril is 165 kJ/mol.[2] When dinitroglycoluril is heated to 243 °C in an inert atmosphere, the two nitrate groups break off and the two central carbon atoms form a double bond.[1]
Sensitivity
The impact sensitivity of dinitroglycoluril was determined using the Bruceton-staircase procedure, which found a h50 of 88 cm. Friction sensitivity was determined by a Julius-Peters apparatus, which found a sensitivity of 25 kg.[2]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads