Top Qs
Timeline
Chat
Perspective
Dioxidanylium
Ion From Wikipedia, the free encyclopedia
Remove ads
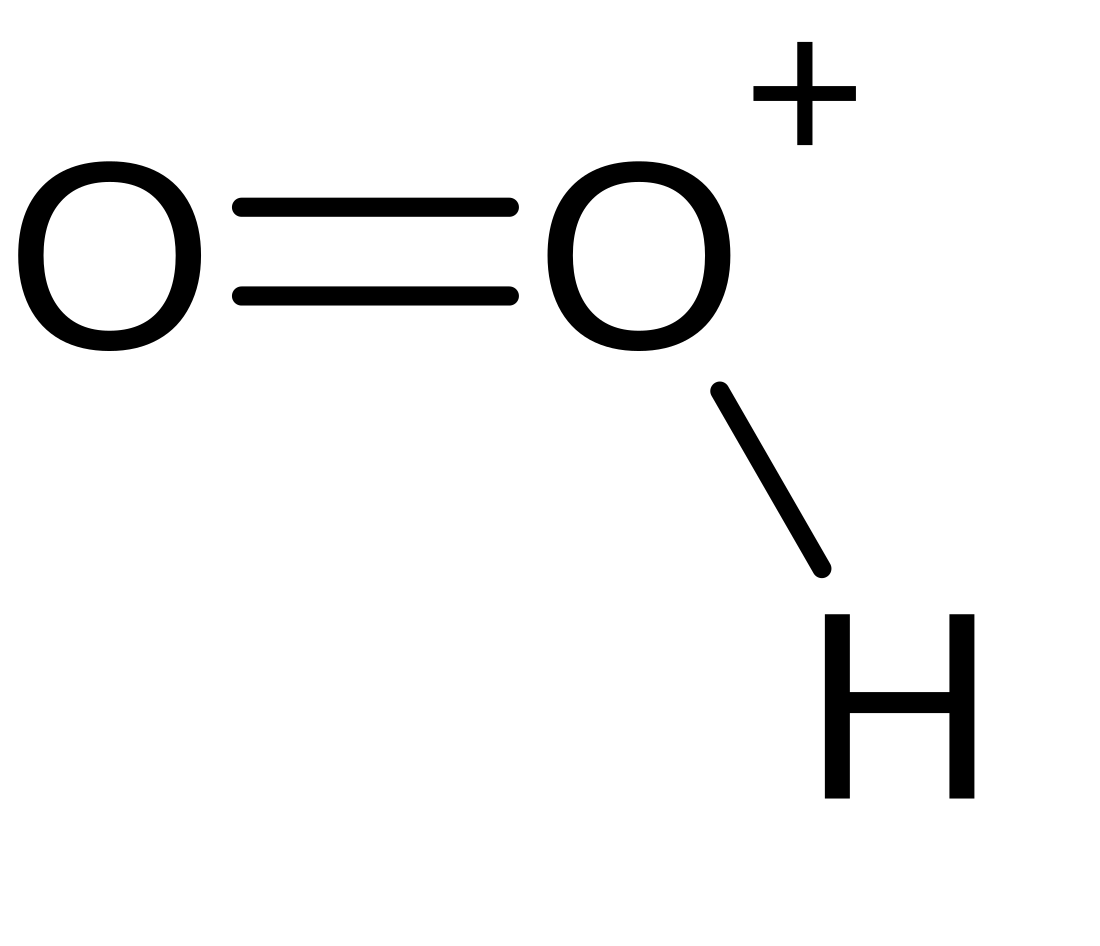
Dioxidanylium, which is protonated molecular oxygen, or just protonated oxygen, is an ion with formula HO+
2.
It is formed when hydrogen containing substances combust, and exists in the ionosphere, and in plasmas that contain oxygen and hydrogen.[2] Oxidation by O2 in superacids could be by way of the production of protonated molecular oxygen.
It is the conjugate acid of dioxygen. The proton affinity of dioxygen (O2) is 4.4 eV.[3]
Remove ads
Significance
Protonated molecular oxygen is of interest in trying to detect dioxygen in space. Because Earth's atmosphere is full of O2, its spectrum from a space object is impossible to observe from the ground. However HO+
2 should be much more detectable.[4]
Formation
Reaction of dioxygenyl O+
2 with hydrogen:[5]
- O+•
2 + H2 → HO+
2 + H•
The reaction of the trihydrogen cation with dioxygen is approximately thermoneutral:[3]
- O2 + H+
3 → HO+
2 + H2
When atomic hydrogen, created in an electric discharge is rapidly cooled with oxygen and condensed in solid neon, several reactive ions and molecules are produced. These include HO2 (hydroperoxyl), HOHOH−, H2O(HO), HOHO− as well as HO+
2.[6] This reaction also forms hydrogen peroxide (H2O2) and hydrogen tetroxide (H2O4).[7]
Remove ads
Properties
In the infrared spectrum HO+
2 the v1 band due to vibrating O–H has a band head at 3016.73 cm−1.[8]
Reactions
A helium complex (He–O2H+) also is known.[8]
HO+
2 appears to react rapidly with hydrogen:[9]
- HO+
2 + H2 → O2 + H+
3
HO+
2 also reacts with dinitrogen and water:[9]
- HO+
2 + H2O → O2 + H3O+
Related
The protonated molecular oxygen dimer HO+
4 has a lower energy than that of protonated molecular oxygen.[3]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads