Top Qs
Timeline
Chat
Perspective
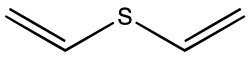
Divinyl sulfide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Divinyl sulfide is the organosulfur compound with the formula S(CH=CH2)2. A colorless liquid with a faint odor, it is found in some species of Allium.[1]
Remove ads
Preparation
Divinyl sulfide is formed from hydrogen sulfide and acetylene.[2] Divinylsulfide can arise when inadvertently when acetylene is generated by hydrolysis of technical-grade calcium carbide contaminated with calcium sulfide.[3]
Divinylsulfide was first prepared in 1920 by the reaction of bis(2-chloroethyl)sulfide with sodium ethoxide:[3]
- (ClCH2CH2)2S + 2 NaOEt → (CH2=CH)2S + 2 EtOH + 2 NaCl
Remove ads
Monovinyl sulfides
With the formula CH2=CHSR, a variety of monovinyl sulfides are known. They can arise by the dehydrohalogenation of -2-haloethyl phenyl sulfides.[4] One example is phenyl vinyl sulfide.[5][6] Alkyl ketones react with thiols in the presence of phosphorus pentoxide to give vinyl sulfides:[7]
- RSH + CH3C(O)R' → CH2=C(SR)R' + H2O
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads