Top Qs
Timeline
Chat
Perspective
Ferrate
From Wikipedia, the free encyclopedia
Remove ads
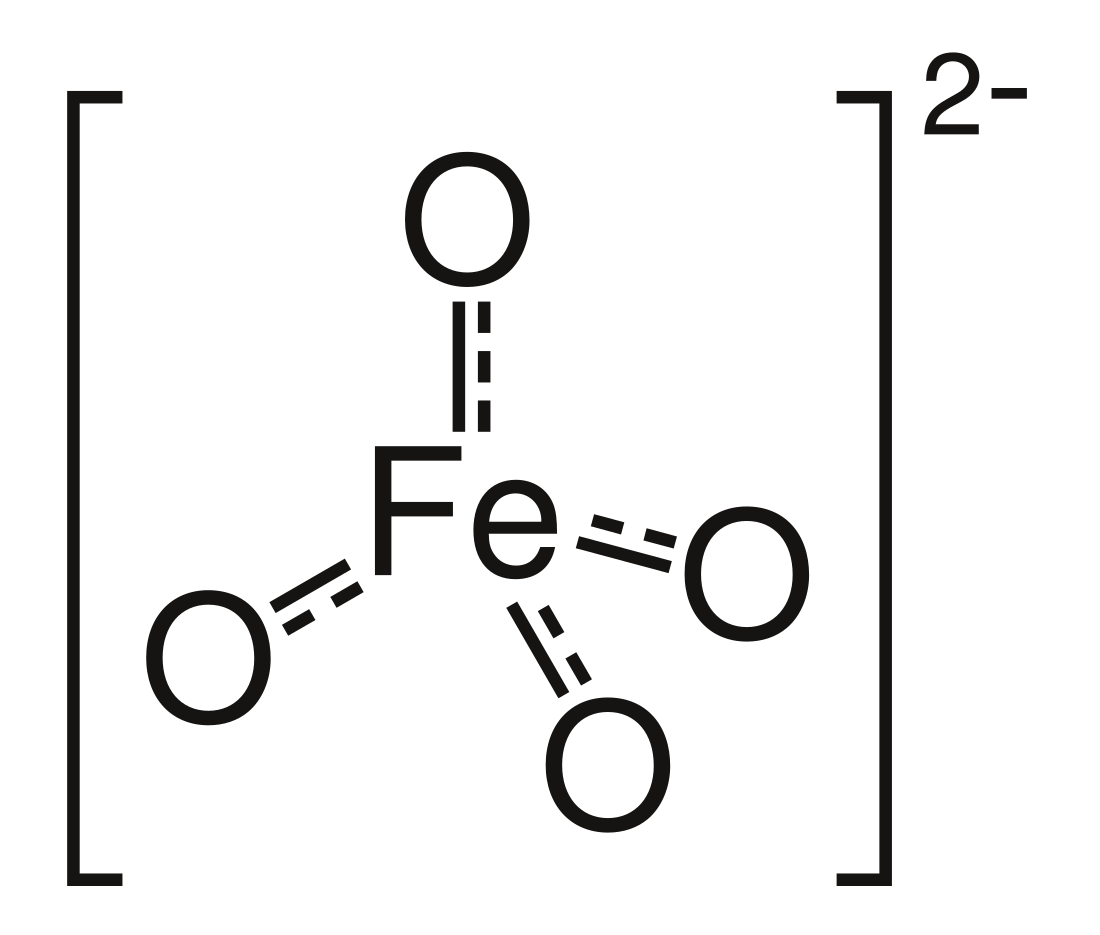
Ferrate loosely refers to a material that can be viewed as containing anionic iron complexes. Examples include tetrachloroferrate ([FeCl4]2−), oxyanions (FeO2−
4), tetracarbonylferrate ([Fe(CO)4]2−), and the organoferrates.[1][page needed] The term ferrate derives from Latin ferrum 'iron'. Some ferrates are called super-iron by some and have uses in battery applications and as an oxidizer.[2][3][4] It can be used to clean water safely from a wide range of pollutants, including viruses, microbes, arsenic, sulfur-containing compounds, cyanides and other nitrogen-containing contaminants, many organic compounds, and algae.[5]
- Ferrates
- Disodium salt of tetracarbonylferrate
- Structure of ferrate(VI), [FeO4]2−
- 1-Butyl-3-methylimidazolium salt of [FeCl4]−
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads