Top Qs
Timeline
Chat
Perspective
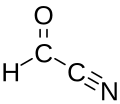
Formyl cyanide
Organic compound (HC(O)C≡N) From Wikipedia, the free encyclopedia
Remove ads
Formyl cyanide is a simple organic compound with the formula HCOCN and structure HC(=O)−C≡N. It is simultaneously a nitrile (R−C≡N) and an aldehyde (R−CH=O). Formyl cyanide is the simplest member of the acyl cyanide family. It is known to occur in space in the Sgr B2 molecular cloud.[1]
Remove ads
Production
Formyl cyanide was first made through methoxyacetonitrile flash vacuum pyrolysis at 600 °C. The same technique with cinnamyloxyacetonitrile[2] or allyloxyacetonitrile also generates formyl cyanide.[3][4]
In molecular clouds, formation of formyl cyanide is speculated to result from formaldehyde and the cyanide radical:[5]
- CH2O + CN• → HCOCN + H•
In Earth's atmosphere, the pollutant acrylonitrile reacts with hydroxyl radical forming formyl cyanide, hydroperoxyl and formaldehyde:[6]
- CH2=CHCN + HO• + 3⁄2 O2 → HOO• + HCOCN + CH2O
Remove ads
Reactions
Formyl cyanide reacts rapidly with trace quantities of water to form formic acid and hydrogen cyanide.[2]
Related
By formally substituting the hydrogen atom, cyanoformyl chloride, ClC(O)CN, and cyanoformyl bromide, BrC(O)CN are obtained.[7]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads