Top Qs
Timeline
Chat
Perspective
Hexaborane(10)
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Hexaborane, also called hexaborane(10) to distinguish it from hexaborane(12) (B6H12), is a boron hydride cluster with the formula B6H10. It is a colorless liquid that is unstable in air.[1]
Remove ads
Structure

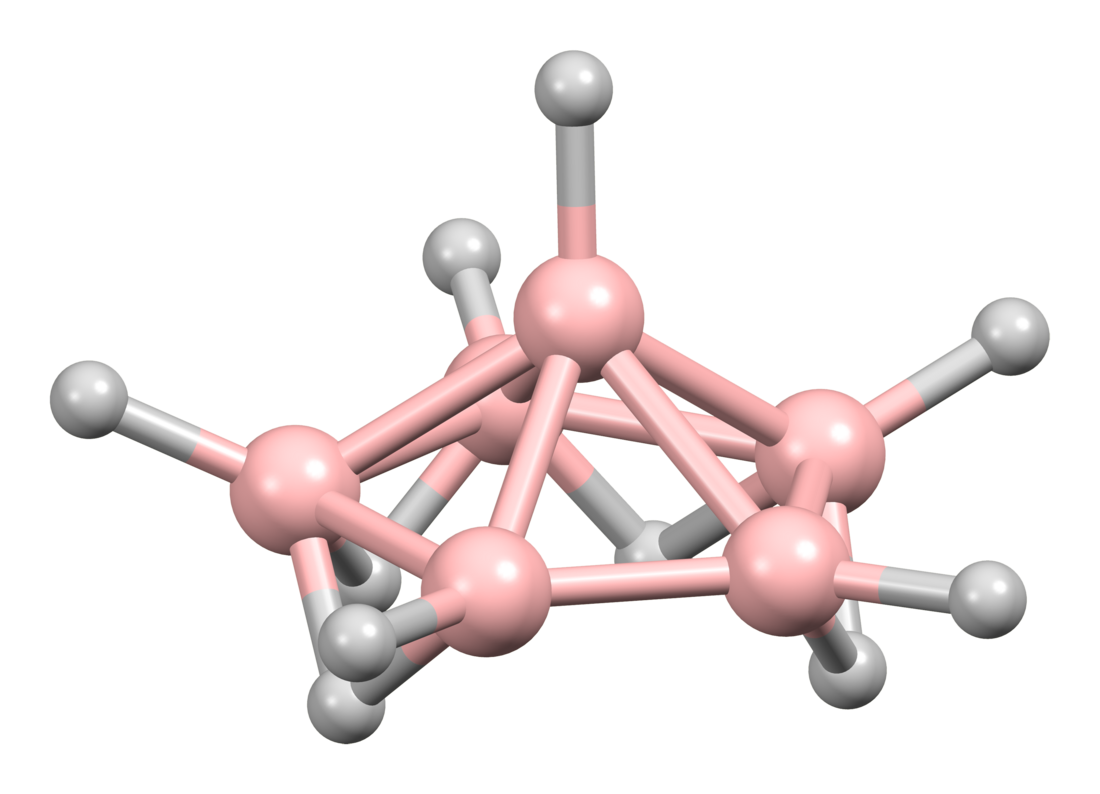
Hexaborane(10) is classified as a nido-cluster.[2]: 152 The boron atoms define a pentagonal pyramid, with four bridging hydrogen atoms and six terminal ones. The point group of the molecule is Cs.[3]
Preparation and reactions
A laboratory route begins with bromination of pentaborane(11) followed by deprotonation of the bromide to give [BrB5H7]−. This anionic cluster is reduced with diborane to give the neutral product:[1]
- K[BrB5H7] + 1/2 B2H6 → KBr + B6H10
It can also be generated by pyrolysis of pentaborane(11).
B6H10 can be deprotonated to give [B6H9]− or protonated to give [B6H11]+.[1] It can act as a Lewis base towards reactive borane radicals, forming various conjuncto-clusters.[2]: 162
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads