Top Qs
Timeline
Chat
Perspective
High-valent iron
Iron in an oxidation state higher than III From Wikipedia, the free encyclopedia
Remove ads
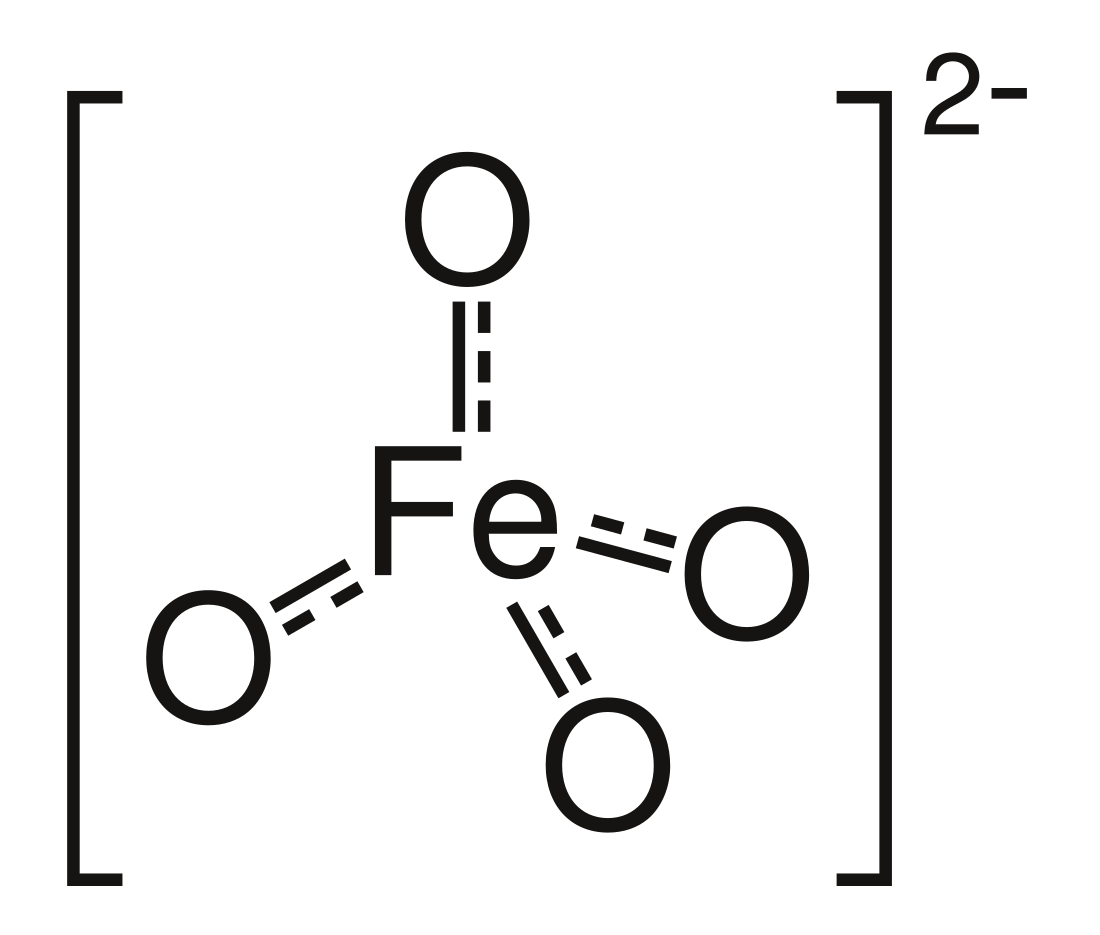
High-valent iron commonly denotes compounds and intermediates in which iron is found in a formal oxidation state > +3 that show a number of bonds > 6 with a coordination number ≤ 6.[according to whom?] The ferrate(VI) ion [FeO4]2− was the first structure in this class synthesized. The synthetic compounds discussed below contain highly oxidized iron in general, as the concepts are closely related.
This article or section possibly contains original synthesis. Source material should verifiably mention and relate to the main topic. (April 2025) |

Remove ads
Oxoiron compounds
Summarize
Perspective
Oxoferryl species are common examples of high-valent iron complexes. Such compounds are prepared by oxidation of ferrous complexes with iodosobenzene:[1][2]
- (mac)FeL2 + OIPh → (mac)Fe=O(L) + IPh + L (mac = tetradentate macrocyclic ligand)
Fe(IV)O

Several syntheses of oxoiron(IV) species have been reported. The simplest are mixed-metal oxides of the form MFeO3, with M=Ba, Ca, or Sr. However, those compounds do not have discrete iron anions.[3]
Isolated oxoiron(IV) species are known with more complicated ligands. These compounds model biological complexes such as cytochrome P450, NO synthase, and isopenicillin N synthase. Two such reported compounds are thiolate-ligated oxoiron(IV) and cyclam-acetate oxoiron(IV).[4]
Thiolate-ligated oxoiron(IV) is formed by the oxidation of a precursor, [FeII(TMCS)](PF6) (TMCS = 1-mercaptoethyl-4,8,11-trimethyl-1,4,8,11-tetraza cyclotetradecane), and 3-5 equivalents of H2O2 at −60 ˚C in methanol. The iron(IV) compound is deep blue in color and shows intense absorption features at 460 nm, 570 nm, 850 nm, and 1050 nm. This species FeIV(=O)(TMCS)+ is stable at −60 ˚C, but decomposition is reported as temperature increases. Compound 2 was identified by Mössbauer spectroscopy, high resolution electrospray ionization mass spectrometry (ESI-MS), X-ray absorption spectroscopy, extended X-ray absorption fine structure (EXAFS), ultraviolet–visible spectroscopy (UV-vis), Fourier-transform infrared spectroscopy (FT-IR), and results were compared to density functional theory (DFT) calculations.[5]
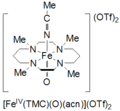
Tetramethylcyclam oxoiron(IV) is formed by the reaction of FeII(TMC)(OTf)2, TMC = 1,4,8,11-tetramethyl-1,4,8,11-tetraazacyclotetradecane; OTf = CF3SO3, with iodosylbenzene (PhIO) in CH3CN at −40 ˚C. A second method for formation of cyclam oxoiron(IV) is reported as the reaction of FeII(TMC)(OTf)2 with 3 equivalents of H2O2 for 3 hours. This species is pale green in color and has an absorption maximum at 820 nm. It is reported to be stable for at least 1 month at −40 ˚C. It has been characterized by Mössbauer spectroscopy, ESI-MS, EXAFS, UV-vis, Raman spectroscopy, and FT-IR.[6]
High-valent iron bispidine complexes can oxidize cyclohexane to cyclohexanol and cyclohexanone in 35% yield with an alcohol to ketone ratio up to 4.[7]
Fe(V)O
FeVTAML(=O), TAML = tetra-amido macrocyclic ligand, is formed by the reaction of [FeIII(TAML)(H2O)](PPh4) with 2-5 equivalents of meta-chloroperbenzoic acid at −60 ˚C in n-butyronitrile. This deep green compound (two λmax at 445 and 630 nm respectively) is stable at 77 K. The stabilization of Fe(V) is attributed to the strong π–donor capacity of deprotonated amide nitrogens.[8]
Fe(VI)O
Ferrate(VI) is found in the inorganic anion [FeO4]2−. It has been isolated as the potassium salt, potassium ferrate. It is a strong water-stable oxidizing agent. Its solutions are stable at high pH.
Remove ads
Nitridoiron and imidoiron compounds
Summarize
Perspective

Nitridoiron[9] and imidoiron[10] compounds are closely related to iron-dinitrogen chemistry.[11] The biological significance of nitridoiron(V) porphyrins has been reviewed.[12][13] A widely applicable method to generate high-valent nitridoiron species is the thermal or photochemical oxidative elimination of molecular nitrogen from an azide complex.
- symbolic oxidative elimination of nitrogen yields a nitridoiron complex; L denotes the supporting ligand.
Fe(IV)N
Several structurally characterized nitridoiron(IV) compounds exist.[14][15][16]
Fe(V)N
The first nitridoiron(V) compound was synthesised and characterized by Wagner and Nakamoto (1988, 1989) using photolysis and Raman spectroscopy at low temperatures.[17][18]
Fe(VI)N
A second FeVI species apart from the ferrate(VI) ion, [(Me3cy-ac)FeN](PF6)2, has been reported. This species, is formed by oxidation followed by photolysis to yield the Fe(VI) species. Characterization of the Fe(VI) complex was done by Mossbauer, EXAFS, IR, and DFT calculations. Unlike the ferrate(VI) ion, compound 5 is diamagnetic.[19]
μ-Nitrido compounds and oxidation catalysis
Bridged μ-nitrido di-iron phthalocyanine compounds such as iron(II) phthalocyanine catalyze the oxidation of methane to methanol, formaldehyde, and formic acid using hydrogen peroxide as sacrificial oxidant.[20][21]
Electronic structure
Nitridoiron(IV) and nitridoiron(V) species were first explored theoretically in 2002.[22]
Remove ads
See also
- Jacobsen's catalyst (high-valent manganese)
References
Further reading
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads