Top Qs
Timeline
Chat
Perspective
Hydroxymethylbilane
Intermediate in the synthesis of porphyrins From Wikipedia, the free encyclopedia
Remove ads
Hydroxymethylbilane, also known as preuroporphyrinogen, is an organic compound that occurs in living organisms during the synthesis of porphyrins, a group of critical substances that include haemoglobin, myoglobin, and chlorophyll. The name is often abbreviated as HMB.
Remove ads
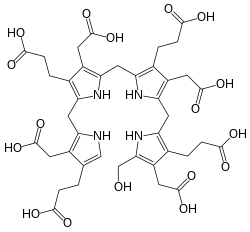
Structure
The compound is a substituted bilane, a chain of four pyrrole rings interconnected by methylene bridges −CH2−. The chain starts with a hydroxymethyl group −CH2−OH and ends with a hydrogen, in place of the respective methylene bridges. The other two carbon atoms of each pyrrole cycle are connected to an acetic acid group −CH2−COOH and a propionic acid group −CH2−CH2−COOH, in that order.[1]
Metabolism
HMB is generated from four molecules of porphobilinogen by the enzyme porphobilinogen deaminase:[2]

The enzyme uroporphyrinogen III synthase closes the chain to form uroporphyrinogen III:[2]

Uroporphyrinogen III is a porphyrinogen, which is a class of compounds with the hexahydroporphine macrocycle. In the absence of the enzyme, the compound undergoes spontaneous cyclization and becomes uroporphyrinogen I.[3][4]
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads