Top Qs
Timeline
Chat
Perspective
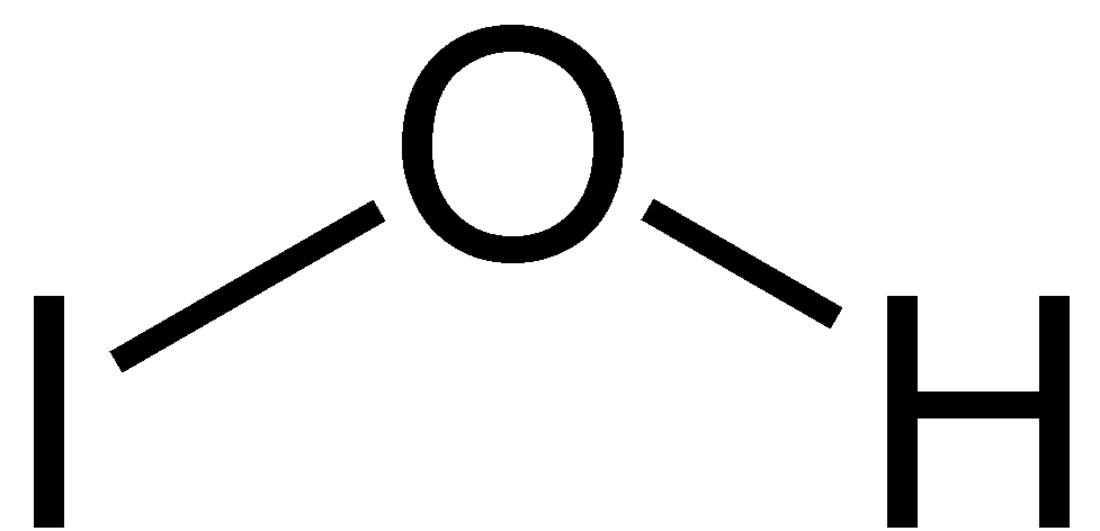
Hypoiodous acid
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Hypoiodous acid is an inorganic compound with the chemical formula HIO. It forms when an aqueous solution of iodine is treated with mercuric or silver salts. It rapidly decomposes by disproportionation:[2]
- 5 HIO → HIO3 + 2 I2 + 2 H2O
Hypoiodous acid is a weak acid with a pKa of about 11. The conjugate base is hypoiodite (IO−). Salts of this anion can be prepared by treating iodine with alkali hydroxides. They rapidly disproportionate to form iodides and iodates,[2] but an iodine–hydroxide mixture can be used an in situ preparation of hypoiodite for other reactions.[3]
Ammonium hypoiodites can be formed by oxidation of the analogous iodide salts. These and also sodium hypoiodite are useful as oxidizing agents for a various types of organic compounds and also for a reaction analogous to the haloform reaction.[3]
Hypoiodite is one of the active oxidizing agents generated by lactoperoxidase as part of the mammalian innate immune system.[4][5]
Remove ads
Other oxyacids
Hypoiodous acid is part of a series of oxyacids in which iodine can assume oxidation states of −1, +1, +3, +5, or +7. A number of neutral iodine oxides are also known.
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads