Top Qs
Timeline
Chat
Perspective
Inositol monophosphatase 1
Protein-coding gene in the species Homo sapiens From Wikipedia, the free encyclopedia
Remove ads
Inositol monophosphatase 1 is an enzyme that in humans is encoded by the IMPA1 gene.[5][6]
Remove ads
Structure
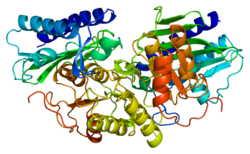
Inositol monophosphatase 1 (IMPA1) is a homodimeric enzyme with each subunit consisting of approximately 277 amino acids and a molecular weight of ~30 kDa.[7] The protein adopts a penta-layered αβαβα core structure, characteristic of the metallophosphatase superfamily, which is also observed in related enzymes like fructose 1,6-bisphosphatase.[8][9] Each monomer's active site contains three magnesium ions arranged in an octahedral coordination geometry, bound by conserved residues including Glu70, Asp90, Asp93, and Asp220.[8][9] Structural studies using X-ray crystallography (e.g., PDB entries 1IMA, 1IMB) reveal that the dimeric arrangement facilitates substrate recognition and catalysis.[9] The enzyme features an Inositol_P domain (Pfam: PF00459), which mediates its magnesium-dependent phosphatase activity and interaction with substrates like myo-inositol monophosphate.[7][8][9] These structural features underpin IMPA1's role in inositol recycling and its sensitivity to lithium inhibition, which occurs through competitive displacement of magnesium ions at the active site.[8]
Remove ads
Function
Inositol monophosphatase 1 (IMPA1) is a magnesium-dependent phosphatase that catalyzes the dephosphorylation of myo-inositol monophosphate to produce free myo-inositol, a crucial precursor for the synthesis of phosphatidylinositol and polyphosphoinositides.[10] These lipids are essential components of cell membranes and play a central role in intracellular signal transduction, particularly in generating the second messengers inositol 1,4,5-trisphosphate and diacylglycerol.[11] IMPA1 exhibits broad substrate specificity, being able to act on various inositol phosphate isomers and other sugar phosphates such as glucose-1-phosphate and fructose-1-phosphate. The enzyme is also notable for being inhibited by lithium at therapeutic concentrations, which underlies its relevance in the treatment of bipolar disorder, as lithium's inhibition of IMPA1 leads to reduced inositol recycling and may modulate phosphoinositide signaling in the brain.[11] Beyond its metabolic role, IMPA1 has been implicated in processes such as autophagy, apoptosis, and cancer progression, highlighting its broader significance in cellular physiology.[6]
Remove ads
Interacting partners
IMPA1 has been shown to interact with Bergmann glial S100B[12] and calbindin.[13][14]
Clinical significance
Summarize
Perspective
As a drug target
Inhibition of IMPA1 can produce pleiotropic effects on cellular function, including alterations in phosphoinositide signalling,[15] autophagy, apoptosis,[16] and other processes.
L-690,330 is a competitive inhibitor of IMPase with high activity in in vitro assays, but it exhibits limited bioavailability in vivo.[17] Owing to its increased specificity relative to lithium, L-690,330 has been widely used to investigate the effects of IMPase inhibition in various cell culture systems. A more cell-permeable prodrug, L-690,488, has also been developed. Treatment of cortical slices with L-690,488 leads to accumulation of inositol, confirming the activity of this inhibitor in tissue.[18]
Bipolar disorder
It was initially observed that several drugs effective in treating bipolar disorder—such as lithium, carbamazepine, and valproic acid—share a common mechanism of action involving enzymes in the phosphatidylinositol signalling pathway.[19] This led to the proposal of the inositol depletion hypothesis as a potential explanation for the pathophysiology of bipolar disorder. However, extensive research has not confirmed this hypothesis, in part because lithium also affects multiple other enzymes within the same pathway, complicating the interpretation of results from in vitro studies.
Intellectual developmental disorder
A homozygous 5-base pair duplication in the IMPA1 gene, resulting in a frameshift and premature stop codon, was identified in a Brazilian family with autosomal recessive intellectual developmental disorder (MRT-59).[20] This mutation, which disrupts neuronal progenitor cell function and impairs differentiation, was absent in control populations and may affect intracellular signaling and neurotransmitter release.[21] Genetic analysis suggests that this syndrome arose in Brazil approximately 200 years ago.[22]
Remove ads
References
Further reading
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads