Top Qs
Timeline
Chat
Perspective
Iron(III) bromide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Iron(III) bromide is the chemical compound with the formula FeBr3. Also known as ferric bromide, this red-brown odorless compound is used as a Lewis acid catalyst in the halogenation of aromatic compounds. It dissolves in water to give acidic solutions.
Remove ads
Structure, synthesis and basic properties
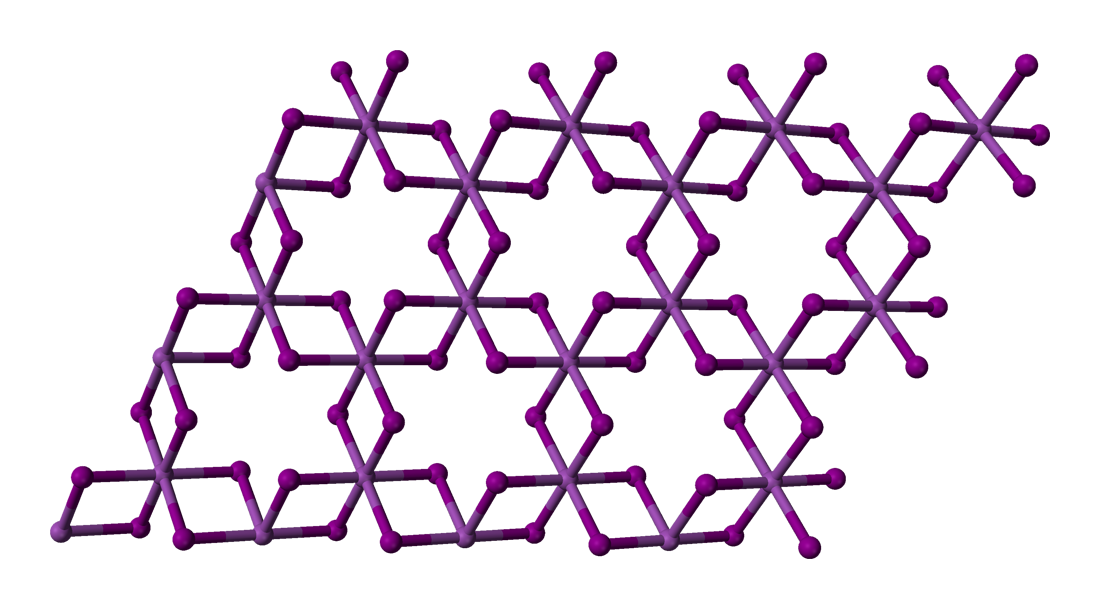
FeBr3 forms a polymeric structure featuring six-coordinate, octahedral Fe centers.[2] Although inexpensively available commercially, FeBr3 can be prepared by treatment of iron metal with bromine:
- 2 Fe + 3 Br2 → 2 FeBr3
Above 200 °C, FeBr3 decomposes to ferrous bromide:
- 2FeBr3 → 2FeBr2 + Br2
Iron(III) chloride is considerably more stable, reflecting the greater oxidizing power of chlorine. FeI3 is not stable, as iron(III) will oxidize iodide ions.
Remove ads
Uses
Ferric bromide is occasionally used as an oxidant in organic chemistry, e.g. for the conversion of alcohols to ketones. It is used as a Lewis acidic catalyst for bromination of aromatic compounds. For the latter applications, it is often generated in situ.[3]
See also
- Iron(II) bromide, the lower bromide of iron
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads