Top Qs
Timeline
Chat
Perspective
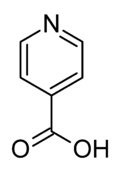
Isonicotinic acid
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Isonicotinic acid or pyridine-4-carboxylic acid is an organic compound with the formula C5H4N(CO2H). It is a derivative of pyridine with a carboxylic acid substituent at the 4-position. It is an isomer of picolinic acid and nicotinic acid, which have the carboxyl group at the 2- and 3-position respectively compared to the 4-position for isonicotinic acid.
Remove ads
Production
On a commercial scale, isonicotinic acid, like other pyridine carboxylic acid is produced by ammoxidation of 4-picoline (4-methylpyridine) followed by hydrolysis of the resulting nitrile:
- NC5H4CH3 + 1.5 O2 + NH3 → NC5H4C≡N + 3 H2O
- NC5H4C≡N + 2 H2O → NC5H4CO2H + NH3
It is also produced by oxidation of 4-picoline with nitric acid.[2]
Derivatives
Isonicotinic acids is a term loosely used for derivatives of isonicotinic acid. Hydrazide derivatives include isoniazid, iproniazid, and nialamide. Amide and ester derivatives include ethionamide and dexamethasone isonicotinate.
Its conjugate base forms coordination polymers[3] and MOFs[4] by binding metal ions through both the N and carboxylate.
See also
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads