Top Qs
Timeline
Chat
Perspective
Isopentane
Chemical compound (C5H12) From Wikipedia, the free encyclopedia
Remove ads
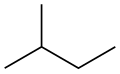
Isopentane, also called methylbutane or 2-methylbutane, is a branched-chain saturated hydrocarbon (an alkane) with five carbon atoms, with formula C
5H
12 or CH(CH
3)
2(C
2H
5).
Isopentane is a volatile and flammable liquid. It is one of three structural isomers with the molecular formula C5H12, the others being pentane (n-pentane) and neopentane (2,2-dimethylpropane).
Isopentane is commonly used in conjunction with liquid nitrogen to achieve a liquid bath temperature of −160 °C. Natural gas typically contains 1% or less isopentane,[3] but it is a significant component of natural gasoline.[4]
Remove ads
History
Although the mixture of pentanes was first isolated from the destructive distillation (pyrolysis) products of the boghead coal by Charles Greville Williams in 1862.[5] In 1864–1865 two chemists tried to extract same hydrocarbons from the Pennsylvanian oil. Carl Schorlemmer noted "that a mere trace of the liquid boiled below 30°C",[6] but the first to properly separate isomers (and thus discover isopentane) was American chemist Cyrus Warren (1824–1891) slightly later, who measured the boiling point of the more volatile one at 30°C.[7]
Remove ads
Nomenclature
The traditional name isopentane, attested in English as early as 1875,[8] was still retained in the 1993 IUPAC recommendations,[9][10] but is no longer recommended according to the 2013 recommendations.[1] The preferred IUPAC name is the systematic name 2-methylbutane. An isopentyl group is a subset of the generic pentyl group. It has the chemical structure -CH3CH2CH(CH3)2.
Uses
Isopentane is used in a closed loop in geothermal power production to drive turbines.[11]
Isopentane is used, in conjunction with dry ice or liquid nitrogen, to freeze tissues for cryosectioning in histology. [12]
Isopentane is a major component (sometimes 30% or more) of natural gasoline, an analog of common petroleum-derived gasoline that is condensed from natural gas.[4] Its share in commercial car fuel is highly variable: 19–45% in 1990s Sweden,[13] 4–31% in 1990s US[14] and 3.6–11% in the US in 2011.[15] It has a substantially higher octane rating (RON 93.7) than n-pentane (61.7), and therefore there is interest in conversion from the latter.[16]
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads