Top Qs
Timeline
Chat
Perspective
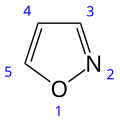
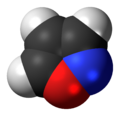
Isoxazole
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Isoxazole is an electron-rich azole with an oxygen atom next to the nitrogen. It is also the class of compounds containing this ring. Isoxazolyl is the univalent functional group derived from isoxazole.
Remove ads
Occurrence
Summarize
Perspective
Isoxazole rings are found in some natural products, such as ibotenic acid and muscimol.
Synthesis
Isoxazole can be synthesised via a variety of methods.[3][4] Examples include via a 1,3-dipolar cycloaddition of nitrile oxides with alkynes; or the reaction of hydroxylamine with 1,3-diketones or derivatives of propiolic acid.[5]
Photochemistry
The photolysis of isoxazole was first reported in 1966.[6] Due to the weak N-O bond, the isoxazole ring tends to collapse under UV irradiation, rearranging to oxazole through azirine intermediate. Meanwhile, the azirine intermediate can react with nucleophiles, especially carboxylic acids. Given the photoreactions, isoxazole group is developed as a native photo-cross-linker for photoaffinity labeling and chemoproteomic studies.[7][8]
Pharmaceuticals and herbicides
Isoxazoles also form the basis for a number of drugs,[9] including the COX-2 inhibitor valdecoxib (Bextra) and a neurotransmitter agonist AMPA. A derivative, furoxan, is a nitric oxide donor. An isoxazolyl group is found in many beta-lactamase-resistant antibiotics, such as cloxacillin, dicloxacillin and flucloxacillin. Leflunomide is an isoxazole-derivative drug. Examples of AAS containing the isoxazole ring include danazol and androisoxazole. A number of pesticides are isoxazoles.[10] Gaboxadol is a conformationally constrained isoxazole derivative of muscimol.

Remove ads
See also
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads