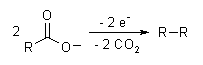Top Qs
Timeline
Chat
Perspective
Kolbe electrolysis
Organic reaction From Wikipedia, the free encyclopedia
Remove ads
The Kolbe electrolysis or Kolbe reaction is an organic reaction named after Hermann Kolbe.[1] The Kolbe reaction is formally a decarboxylative dimerisation of two carboxylic acids (or carboxylate ions). The overall reaction is:
Mechanism and side-reactions
Summarize
Perspective
The reaction mechanism involves a two-stage radical process: electrochemical oxidation first gives a alkylcarboxyl radical, which decarboxylates almost immediately to give an alkyl radical intermediate. The alkyl radicals which combine to form a covalent bond.[2] As an example, electrolysis of acetic acid yields ethane and carbon dioxide:
- CH3COOH → CH3COO− → CH3COO· → CH3· + CO2
- 2CH3· → CH3CH3
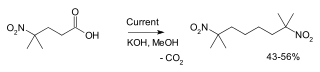
Another example is the synthesis of 2,7-dimethyl-2,7-dinitrooctane from 4-methyl-4-nitrovaleric acid:[3]
Other compounds can trap the radicals formed by decarboxylation, and the Kolbe reaction has also been occasionally used in cross-coupling reactions. If a mixture of two different carboxylates are used, the radical cross-coupling reaction generally gives all combinations of them:[4]
- R1COO− + R2COO− → R1−R1 and/or R1−R2 and/or R2−R2
The reaction process can be enhanced and the Hofer–Moest reaction alternative suppressed, by performing the reaction under weakly acidic conditions in protic solvents, and using a high current density and a platinum anodic electrode.[4]
In 2022, it was discovered that the Kolbe electrolysis is enhanced if an alternating square wave current is used instead of a direct current.[5][6]
Hofer–Moest reaction
In the Hofer–Moest reaction, the alkyl radical undergo further oxidation to form a carbocation, rather than coupling with another alkyl radical, which then reacts with an available nucleophile.[7] The Hofer–Moest reaction, rather than Kolbe radical-coupling, always occurs if the carboxylic acid bears a carbocation-stabilizing side-substituent at the α position, but only sometimes otherwise.[4]
Remove ads
Applications
Kolbe electrolysis has a few industrial applications.[8] The reaction typically yields <50%.[4]
In one example, sebacic acid has been produced commercially by Kolbe electrolysis of adipic acid.[9]
Kolbe electrolysis has been examined for converting biomass into biodiesel[10][11] and for grafting of carbon electrodes.[12][13]
See also
References
Further reading
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads