Top Qs
Timeline
Chat
Perspective
Lead tetrafluoride
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
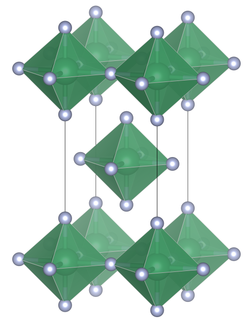
Lead tetrafluoride is a compound of lead and fluorine. The yellow solid (melting point 600 °C) is the only room-temperature stable tetrahalide of lead.[3] Lead tetrafluoride is isostructural with tin(IV) fluoride and contains planar layers of octahedrally coordinated lead, where the octahedra share four corners and there are two terminal, unshared, fluorine atoms trans to one another.[4]
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads