Top Qs
Timeline
Chat
Perspective
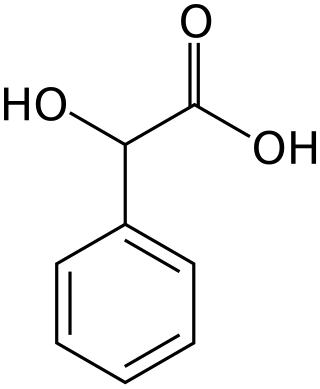
Mandelic acid
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Mandelic acid is an aromatic alpha hydroxy acid (AHA) with the molecular formula C6H5CH(OH)CO2H. It is a white crystalline solid that is soluble in water and polar organic solvents. Its principal use in organic synthesis is as a useful precursor to various drugs.
Remove ads
Properties
At room temperature, mandelic acid is a white or colorless solid with a faint odor.[4] It is highly soluble in diethyl ether[5] but less so in water and ethanol.[2][4][6] It is insoluble in petroleum ether.[5]
The molecule is chiral. The racemic mixture is known as paramandelic acid.
Isolation, synthesis, occurrence
Summarize
Perspective
Mandelic acid was discovered in 1831 by the German pharmacist Ferdinand Ludwig Winckler (1801–1868) while heating amygdalin, an extract of bitter almonds, with diluted hydrochloric acid. The name is derived from the German "Mandel" for "almond".[7]
Mandelic acid is usually prepared by the acid-catalysed hydrolysis of mandelonitrile,[8] which is the cyanohydrin of benzaldehyde and can be synthesized in various ways:[9]
Alternatively, it can be prepared by a substitution reaction from bromophenylacetic acid, as well as by hydrolysis routes starting from various α,α-dihaloacetophenones.[10] It also arises by an isomerization reaction upon heating phenylglyoxal with various alkalis.[11][12]
Biosynthesis
Mandelic acid is a substrate or product of several biochemical processes called the mandelate pathway. Mandelate racemase interconverts the two enantiomers via a pathway that involves cleavage of the alpha-CH bond. Mandelate dehydrogenase is yet another enzyme on this pathway.[13] Mandelate also arises from trans-cinnamate via phenylacetic acid, which is hydroxylated.[14] Phenylpyruvic acid is another precursor to mandelic acid.
Derivatives of mandelic acid are formed as a result of metabolism of adrenaline and noradrenaline by monoamine oxidase and catechol-O-methyl transferase. The biotechnological production of 4-hydroxy-mandelic acid and mandelic acid on the basis of glucose was demonstrated with a genetically modified yeast Saccharomyces cerevisiae, in which the hydroxymandelate synthase naturally occurring in the bacterium Amycolatopsis was incorporated into a wild-type strain of yeast, partially altered by the exchange of a gene sequence and expressed.[15]
It also arises from the biodegradation of styrene[16] and ethylbenzene, as detected in urine.
Remove ads
Uses
Summarize
Perspective
Cosmetics
Mandelic acid can be a component of chemical face peels analogous to other alpha hydroxy acids.[2] Mandelic acid is one of the most common chemical components of the "superficial peel" class, which destroy all or part of the epidermis while remaining safe to use on all Fitzpatrick skin types.[17][18][19] The American Academy of Dermatology says there is insufficient evidence to recommend chemical peels (including those with mandelic acid) as a treatment for acne vulgaris.[20] While noting it was widely used in cosmetic products, known to be effective, and frequently prescribed for acne, mandelic acid was among the ingredients not recommended for acne in a 2025 Journal of the American Academy of Dermatology literature review's expert panel, because it is rarely covered by insurance and more costly than treatments with similar effects such as vitamin A derivatives.[21]
Pharmaceuticals
Mandelic acid is widely used for pharmaceuticals as a precursor to various drugs.[22][better source needed]
The drugs cyclandelate and homatropine[23] are esters of mandelic acid.[citation needed] Homatropine dilates eyes for eye exams and wears off quickly.[23] This effect was discovered by chemist Alfred Ladenburg in 1880 and was preferred because the previous mixture caused blurry vision for days. Mandelic acid replaced tropic acid in the synthesis of homatropine.[23][24][25]
Toxicology
Mandelic acid levels in human urine are a standard biomarker for styrene exposure in industrial hygiene. The unstable metabolite styrene-(7,8)-oxide (styrene oxide) is oxidized into mandelic acid and phenylglyoxylic acid, then exits the body in urine.[26][27] It is also a biomarker for ethylbenzene exposure.[26][28][29] Daily end-of-shift urine collection to monitor styrene exposure is recommended by American Conference of Governmental Industrial Hygienists (ACGIH).[30]
History
Mandelic acid was discovered in 1831 by the German pharmacist Ferdinand Ludwig Winckler (1801–1868) while heating amygdalin, an extract of bitter almonds, with diluted hydrochloric acid. The name is derived from the German "Mandel" for "almond".[7]
The short-acting eye dilation effect as part of homatropine was discovered by chemist Alfred Ladenburg in 1880. Mandelic acid then replaced tropic acid in the synthesis of homatropine.[31][23][24]
Takeru Higuchi and Roy Kuramoto demonstrated one of the earliest forms of pharmaceutical cocrystals in studies published during 1954 that involved mandelic acid.[32]
Remove ads
Safety and handling
Mandelic acid is moderately toxic if ingested.[4][33] It is poisonous if injected.[33] Frequent absorption can result in kidney irritation.[33] When burning, mandelic acid emits acrid smoke and fumes.[33]
Exposing the white crystal form to light will darken to brown and decompose the crystals over time.[4][5][33]
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads