Top Qs
Timeline
Chat
Perspective
Metaphosphate
From Wikipedia, the free encyclopedia
Remove ads
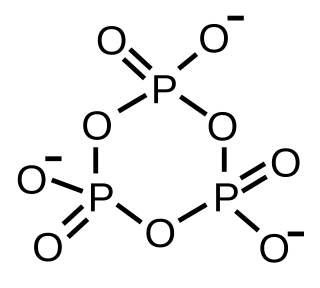
A metaphosphate ion is an oxyanion that has the empirical formula PO−
3.[1] It was first postulated in 1955[2] but was not observed until 1979, when it was detected by mass spectrometry.[3] Metaphosphate is an intermediate in the hydrolysis of phosphate esters but it is difficult to isolate, as it readily hydrolyses to from a dihydrogen phosphate ion ([H
2PO
4]−
) and tends to self-react in the absence of water to form rings or infinite chains:[4] These species are also called metaphosphates and are generally stable, with some such as sodium trimetaphosphate being produced on an industrial scale.
Metaphosphates can be used as an alternative of white phosphorus in organic syntheses.[5]
Remove ads
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads
