Top Qs
Timeline
Chat
Perspective
Methanetetracarboxylate
Ion From Wikipedia, the free encyclopedia
Remove ads
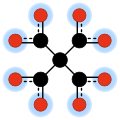
Methanetetracarboxylate is the tetraanion with formula C(CO2)4−4. It has four carboxylate groups attached to a central carbon atom; so it has the same carbon backbone as neopentane. It is an oxocarbon anion, that is, consists only of carbon and oxygen.
This article needs attention from an expert in Chemicals. Please add a reason or a talk parameter to this template to explain the issue with the article. (April 2025) |
The term is also used for any salt with that anion; or for any ester with the C(−C(=O)−O−)4 moiety.[1]
The salts and esters are relatively uncommon, and their uses appear to be limited to chemical research. Sodium methanetetracarboxylate Na4C(CO2)4 can be obtained by oxidation of pentaerythritol C(CH2OH)4 with oxygen in sodium hydroxide solution at pH 10 and about 60 °C, in the presence of palladium as a catalyst.[2]
The anion can be seen as the result of removing four protons from methanetetracarboxylic acid, a hypothetical organic compound with formula H4C5O8 or C(COOH)4. This acid has not been synthesised (as of 2009), and is believed to be unstable, but its ethyl ester, tetraethyl methanetetracarboxylate, C(COOCH2CH3)4, is a specialty chemical and has been used in organic synthesis.[3]
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads