Top Qs
Timeline
Chat
Perspective
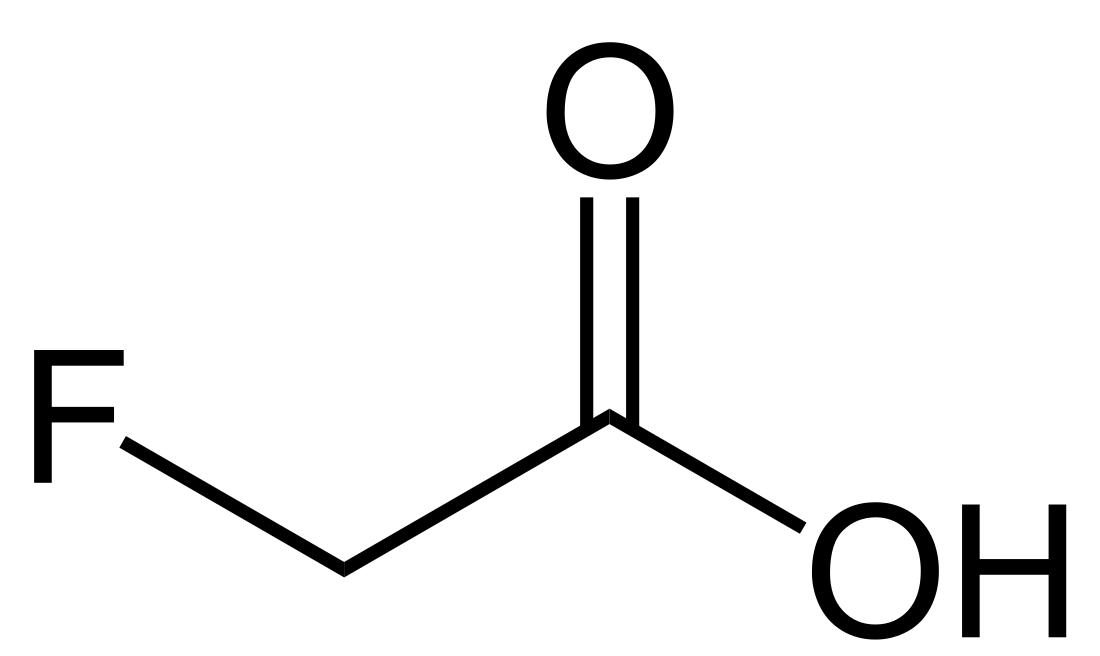
Fluoroacetic acid
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Fluoroacetic acid is an organofluorine compound with the chemical formula FCH2CO2H. It is a colorless solid that is noted for its relatively high toxicity.[1] The conjugate base, fluoroacetate occurs naturally in at least 40 plants in Australia, Brazil, and Africa. It is one of only five known organofluorine-containing natural products.[2]
Remove ads
Toxicity
Fluoroacetic acid is a harmful metabolite of some fluorine-containing drugs (median lethal dose, LD50 = 10 mg/kg in humans). The most common metabolic sources of fluoroacetic acid are fluoroamines and fluoroethers. Fluoroacetic acid can disrupt the Krebs cycle.[3] The metabolite of fluoroacetic acid is Fluorocitric acid and is very toxic because it is not processable using aconitase in the Krebs cycle (where fluorocitrate takes place of citrate as the substrate). The enzyme is inhibited and the cycle stops working.[4]
In contrast with fluoroacetic acid, difluoroacetic acid and trifluoroacetic acid are far less toxic. Its pKa is 2.66[contradictory], in contrast to 1.24 and 0.23 for the respective di- and trifluoroacetic acid.[5]
Remove ads
Uses
Fluoroacetic acid is used to manufacture pesticides especially rodenticides such as sodium fluoroacetate (compound 1080). The overall market is projected to rise at a considerable rate during the forecast period, 2021 to 2027.[6]
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads