Top Qs
Timeline
Chat
Perspective
Nitrosyl fluoride
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Nitrosyl fluoride (NOF) is a covalently bonded nitrosyl compound.
This article relies largely or entirely on a single source. (October 2023) |
Remove ads
Physical properties
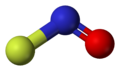
The compound is a colorless gas, with bent molecular shape.[1] The VSEPR model explains this geometry via a lone-pair of electrons on the nitrogen atom.
Chemistry
Nitrosyl fluoride is typically produced by direct reaction of nitric oxide and fluorine, although halogenation with a perfluorinated metal salt is also possible. The compound is a highly reactive fluorinating agent that converts many metals to their fluorides, releasing nitric oxide in the process:
- n NOF + M → MFn + n NO
For this reason, aqueous NOF solutions are, like aqua regia, powerful solvents for metals.[1]
Absent an oxidizable metal, NOF reacts with water to form nitrous acid, which then disproportionates to nitric acid:
- NOF + H2O → HNO2 + HF
- 3 HNO2 → HNO3 + 2 NO + H2O
These reactions occur in both acidic and basic solutions.[1]
Nitrosyl fluoride also forms salt-like adducts with Lewis-acidic fluorides; for example, BF3 reacts to give NOBF4. Similarly, the compound nitrosylates compounds with a free proton; thus alcohols convert to nitrites:[1]
- ROH + NOF → RONO + HF
Remove ads
Uses
Nitrosyl fluoride is used as a solvent and as a fluorinating and nitrating[dubious – discuss] agent in organic synthesis.[citation needed]
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads