Top Qs
Timeline
Chat
Perspective
Norsteroid
From Wikipedia, the free encyclopedia
Remove ads
Norsteroids (nor-, L. norma, from "normal" in chemistry, indicating carbon removal) are a structural class of steroids that have had an atom or atoms (typically carbon) removed, biosynthetically or synthetically, from positions of branching off of rings or side chains (e.g., removal of methyl groups), [1] or from within rings of the steroid ring system.[2][3] Norsteroids are systematically classified into four categories, A-, B-, C-, and D-norsteroids depending on ring if modified. Natural sources of norsteroids include but are not limited to sponges, corals, terrestrial plants, fungi, and bacteria. [4]For instance, 19-norsteroids (e.g., 19-norprogesterone) constitute an important class of natural and synthetic steroids derived by removal of the methyl group of the natural product progesterone; the equivalent change between testosterone and 19-nortestosterone (nandrolone) is illustrated below.
This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages)
|
Remove ads
Examples
Norsteroid examples include: 19-norpregnane (from pregnane), desogestrel, ethylestrenol, etynodiol diacetate, ethinylestradiol, gestrinone, levonorgestrel, norethisterone (norethindrone), norgestrel, norpregnatriene (from pregnatriene), quinestrol, 19-norprogesterone (from a progesterone), Nomegestrol acetate,[5][non-primary source needed] 19-nortestosterone (from a testosterone), and norethisterone acetate.[5][non-primary source needed]
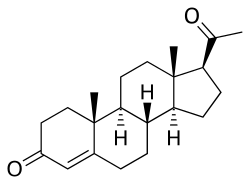 |  | |
| progesterone | 19-norprogesterone | |
 |  | |
| testosterone | nandrolone (nortestosterone) |
Remove ads
Ganonorsterone A
In 2023 under the guidance of the Zhao research group from the Henan University of Chinese Medicine, in Zhengzhou, China, they discovered the compound Ganonorsterone A. Obtained as a colorless oil, this compound was proved to be a dicyclic norsteroid. Using analysis of Nuclear magnetic resonance (NMR) data along with High-performance liquid chromatography (HPLC), and Electronic Circular Dichroism (ECD) it was concluded the formula of the compound to be C19H28O2. This compound exhibited moderate inhibition on Nitric Oxide production in macrophages demonstrating potential anti-inflammatory activity; however, no cytotoxicity, or the ability to damage or kill cells, was discovered against five human cancer cell lines including but not limited to HL-60 (Acute promyelocytic leukemia), SMMC-7721 (Hepatocellular carcinoma), A549 (Lung adenocarcinoma), MCF-7 (Breast adenocarcinoma), and SW-480 (Colorectal adenocarcinoma). [6]
Remove ads
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads