Top Qs
Timeline
Chat
Perspective
Onapristone
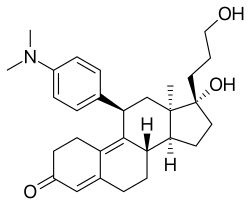
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Onapristone (INN) (developmental code names ZK-89299, ZK-299) is a synthetic and steroidal antiprogestogen with additional antiglucocorticoid activity which was developed by Schering[1] and described in 1984 but was never marketed.[2][3] It is a silent antagonist of the progesterone receptor (PR), in contrast to the related antiprogestogen mifepristone (which is a weak partial agonist of the receptor).[4] Moreover, compared to mifepristone, onapristone has reduced antiglucocorticoid activity, shows little antiandrogenic activity, and has 10- to 30-fold greater potency as an antiprogestogen.[4] The medication was under development for clinical use, for instance in the treatment of breast cancer and as an endometrial contraceptive, but was discontinued during phase III clinical trials in 1995 due to findings that liver function abnormalities developed in a majority patients.[5][6][7]
Onapristone has been found to be effective in the treatment of breast cancer.[8][5][9]
As of 2016, onapristone has re-emerged and is under development for the treatment of prostate cancer, currently in phase II clinical trials.[10] It was also under development for the treatment of endometrial cancer, breast cancer, ovarian cancer, and uterine cancer, but was discontinued for these indications in favor of focusing on prostate cancer.[10]
Remove ads
Synthesis
Reaction of the steroid derivative (1) and the Grignard reagent 4-(dimethylamino)phenylmagnesium bromide (2) gives (3) by vinylogous addition to the epoxide. Oxidation of the alcohol group in the five-membered ring to a ketone gives compound (4). Irradiation of this material for 16 minutes with a mercury lamp results in the methyl group adjacent to the ketone changing from the beta to the alpha configuration, giving (5). Alkynylation with the anion formed from the acetylene derivative (6) using butyllithium gives (7). Catalytic hydrogenation to convert the alkyne group to an alkyl group, followed by acid treatment to remove the protecting groups yielded onapristone.[11][12]
Remove ads
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads