Top Qs
Timeline
Chat
Perspective
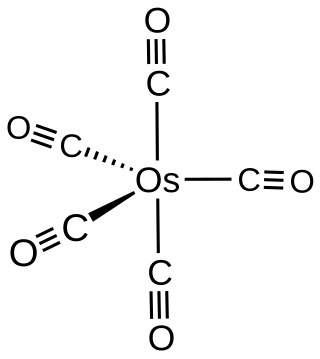
Osmium pentacarbonyl
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Osmium pentacarbonyl is the organoosmium compound with the formula Os(CO)5. It is the simplest isolatable carbonyl complex of osmium. Osmium pentacarbonyl is a colorless volatile liquid that is obtained by treating solid triosmium dodecacarbonyl under 200 atmospheres of carbon monoxide at 280-290 °C. In contrast, also at 200 atm of CO, solid Ru3(CO)12 converts to Ru(CO)5 at milder temperature of 160 °C.[1]
Remove ads
Reactions

Samples of Os(CO)5 convert back to the trioosmium cluster upon heating to 80 °C. The analogous conversion of Ru(CO)5 back to Ru3(CO)12 occurs at room temperature.[1] Chlorination of the pentacarbonyl gives a cationic pentacarbonyl complex:[1]
- Os(CO)5 + Cl2 → [Os(CO)5Cl]+Cl−
Upon UV irradiation, hexane solutions of the pentacarbonyl react with ethylene to give mono-, di-, and trisubstituted derivatives:[3]
- Os(CO)5 + n C2H4 → Os(CO)5-n(C2H4)n + n CO (n = 1,2,3)
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads