Top Qs
Timeline
Chat
Perspective
Panthenol
Pair or mixture of stereoisomers. From Wikipedia, the free encyclopedia
Remove ads
Panthenol (also called pantothenol) is the alcohol analog of pantothenic acid (vitamin B5), and is thus a provitamin of B5. In organisms, it is quickly oxidized to pantothenic acid. It is a viscous transparent liquid at room temperature. Panthenol is used in pharmaceutical and children's products as a moisturizer and to hasten wound healing.
Remove ads
Adverse effects
Panthenol is generally well-tolerated. In rare cases, skin irritation causing contact dermatitis and contact allergies have been reported.[2][3]
Pharmacology
Panthenol readily penetrates into the skin and mucous membranes (including the intestinal mucosa), where it is quickly oxidized to pantothenic acid. Pantothenic acid is extremely hygroscopic.[4] It is also used in the biosynthesis of coenzyme A, which plays a role in a wide range of enzymatic reactions and in cell growth.[2][3]
Physical and chemical properties

Panthenol is an odourless, slightly bitter, highly viscous, transparent, and colourless liquid at room temperature,[5] but salts of pantothenic acid (for example sodium pantothenate) are powders that are typically white. It is easily soluble in water and alcohol, moderately soluble in diethyl ether, soluble in chloroform (1:100),[5] in propylene glycol, and slightly soluble in glycerin.
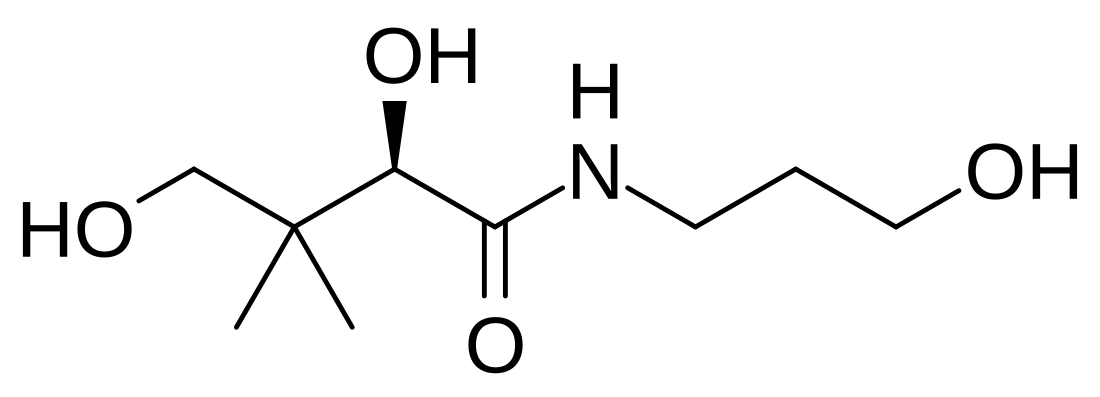
Panthenol's expanded chemical formula is HO–CH2–C(CH3)2–CH(OH)–CONH–CH2CH2CH2–OH.
Stereochemistry
Panthenol comes in two enantiomers: D, and L. Only D-panthenol (dexpanthenol) is biologically active, however both forms have moisturizing properties. For cosmetic use, panthenol comes either in D form, or as a racemic mixture of D and L (DL-panthenol).
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads