Top Qs
Timeline
Chat
Perspective
Peroxynitrite
Ion From Wikipedia, the free encyclopedia
Remove ads
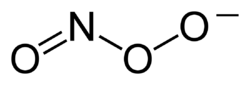
Peroxynitrite (sometimes called peroxonitrite) is an ion with the formula ONOO−. It is a structural isomer of nitrate, NO−
3. Peroxynitrite is a potent reactive nitrogen species and is highly cytotoxic.[1]
Remove ads
Preparation
Peroxynitrite can be prepared by the reaction of superoxide with nitric oxide:[2][3][4]
- NO + O−2 → NO(O2)−
It is prepared by the reaction of hydrogen peroxide with nitrite:[5]
- H2O2 + NO−
2 → ONOO− + H2O
Its presence is indicated by the absorbance at 302 nm (pH 12, ε302 = 1670 M−1 cm−1).
Reactions
Peroxynitrite is weakly basic with a pKa of ~6.8.
It is reactive toward DNA and proteins.
ONOO− reacts nucleophilically with carbon dioxide.[6] In vivo, the concentration of carbon dioxide is about 1 mM, and its reaction with ONOO− occurs quickly. Thus, under physiological conditions, the reaction of ONOO− with carbon dioxide to form nitrosoperoxycarbonate (ONOOCO−
2) is by far the predominant pathway for ONOO−. ONOOCO−
2 homolyzes to form carbonate radical and nitrogen dioxide, again as a pair of caged radicals. Approximately 66% of the time, these two radicals recombine to form carbon dioxide and nitrate. The other 33% of the time, these two radicals escape the solvent cage and become free radicals. It is these radicals (carbonate radical and nitrogen dioxide) that are believed to cause peroxynitrite-related cellular damage.
Remove ads
Peroxynitrous acid
Its conjugate acid peroxynitrous acid is highly reactive, although peroxynitrite is stable in basic solutions.[7][8]
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads