Top Qs
Timeline
Chat
Perspective
Phosphorus pentabromide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
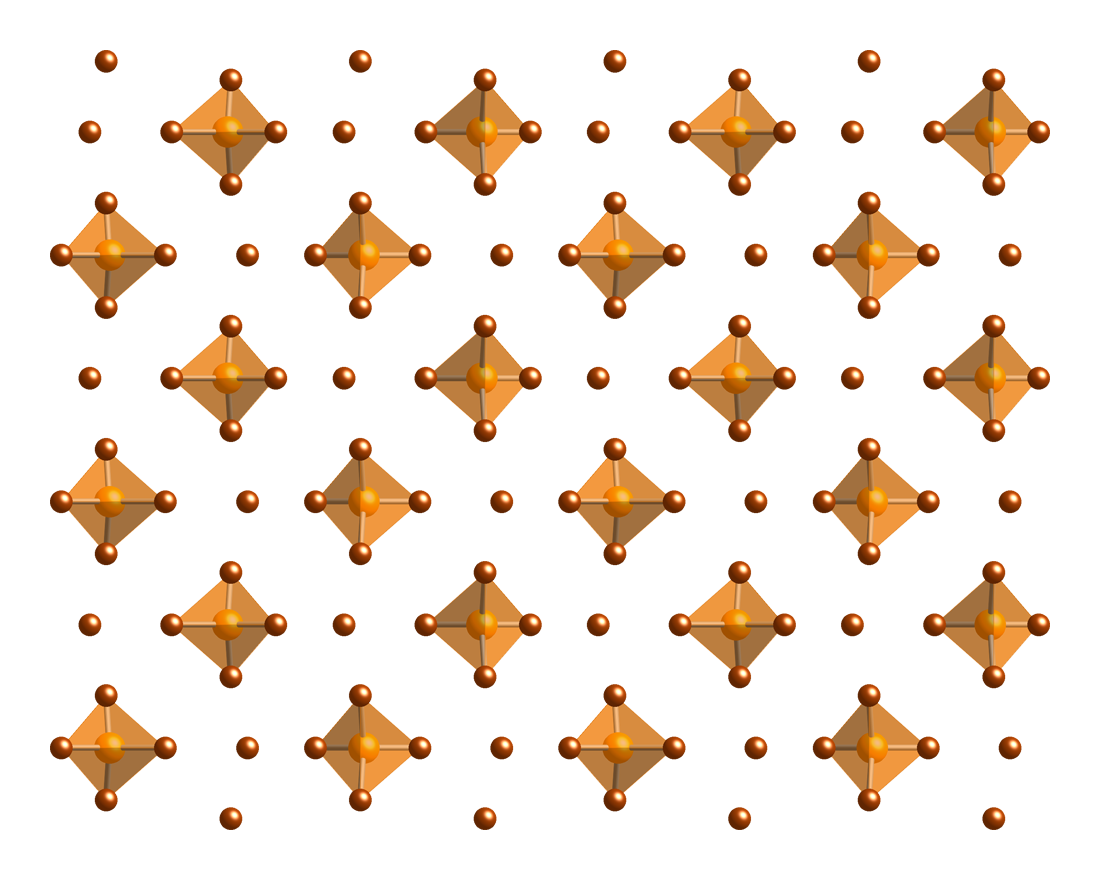
Phosphorus pentabromide is a reactive, yellow solid of formula PBr5, which has the structure [PBr4]+Br− (tetrabromophosphonium bromide) in the solid state but in the vapor phase is completely dissociated to PBr3 and Br2. Rapid cooling of this phase to 15 K leads to formation of the ionic species phosphorus heptabromide (tetrabromophosphonium tribromide [PBr4]+[Br3]−).[2]
It can be used in organic chemistry to convert carboxylic acids to acyl bromides. It is highly corrosive. It strongly irritates skin and eyes.[1] It decomposes above 100 °C to give phosphorus tribromide and bromine:[3]
Reversing this equilibrium to generate PBr5 by addition of Br2 to PBr3 is difficult in practice because the product is susceptible to further addition to yield phosphorus heptabromide [PBr4]+[Br3]−.[4]
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads