Top Qs
Timeline
Chat
Perspective
Pinene
Oily organic chemical found in plants From Wikipedia, the free encyclopedia
Remove ads
Pinene is a collection of unsaturated bicyclic monoterpenes. Two geometric isomers of pinene are found in nature, α-pinene and β-pinene. Both are chiral, and both contain a strained four-membered ring. As the name suggests, pinenes are found in pines. Specifically, pinene is the major component of the liquid extracts of conifers.[3] Pinenes are also found in many non-coniferous plants such as camphorweed (Heterotheca)[4] and big sagebrush (Artemisia tridentata).
Remove ads
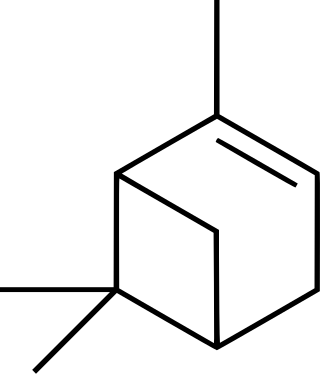
Isomers
| skeletal formula |  |  |  |  |
| perspective view | X |  | X | 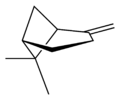 |
| ball-and-stick model |  | 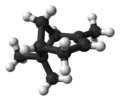 | X |  |
| name | (1R)-(+)-α-pinene | (1S)-(−)-α-pinene | (1R)-(+)-β-pinene | (1S)-(−)-β-pinene |
| CAS number | 7785-70-8 | 7785-26-4 | 19902-08-0 | 18172-67-3 |
Biosynthesis
α-Pinene and β-pinene are both produced from geranyl pyrophosphate, via cyclisation of linaloyl pyrophosphate followed by loss of a proton from the carbocation equivalent. Researchers at the Georgia Institute of Technology and the Joint BioEnergy Institute have been able to synthetically produce pinene with a bacterium.[5]

Plants
Alpha-pinene is the most widely encountered terpenoid in nature[6] and is highly repellent to insects.[7]
Alpha-pinene appears in conifers and numerous other plants.[8] Pinene is a major component of the essential oils of Sideritis spp. (ironwort)[9] and Salvia spp. (sage).[10] Cannabis also contains alpha-pinene[8] and beta-pinene, as well as the dried finished flowers known as marijuana.[11] Resin from Pistacia terebinthus (commonly known as terebinth or turpentine tree) is rich in pinene. Pine nuts produced by pine trees contain pinene.[8]
Makrut lime fruit peel contains an essential oil comparable to lime fruit peel oil; its main components are limonene and β-pinene.[12] Essential oils of other citrus fruits like oranges and lemons also include racemic mixtures of alpha- and beta-pinene. Oils of herbs like rosemary and basil also contain racemic mixtures of pinene.
The racemic mixture of the two forms of pinene is found in some oils like eucalyptus oil.[13]
Reactions
α-Pinene
Selective oxidation of α-pinene occurs at the allylic position to give verbenone, along with pinene oxide, as well as verbenol and its hydroperoxide.[14][15]

Pinene's chiral bicyclic nature and availability makes it an attractive chiral starting material for complex syntheses. For example, pinene was explored by Paul Wender as a starting material for his total synthesis of taxol.[16]
α-Pinene can be converted to camphor by way of isobornyl acetate. Hydrogenation of pinene gives pinane, precursor to a useful pinanehydroperoxide. The hydroboration of α-pinene has been extensively examined. With borane-dimethylsulfide, two equivalents of α-pinene react to give (diisopinocampheyl)borane.[17] Reaction with 9-BBN gives the reagent called alpine borane. This sterically crowded chiral trialkylborane can stereoselectively reduce aldehydes in what is known as the Midland Alpine borane reduction.[18]
β-pinene
β-pinene can be converted to α-pinene in the presence of strong bases,[19] or pyrolysed to produce myrcene at 400 °C.
Remove ads
Use
Pinenes, especially α, are the primary constituents of turpentine, a nature-derived solvent and fuel.[3]
The use of pinene as a biofuel in spark ignition engines has been explored.[20] Pinene dimers have been shown to have heating values comparable to the jet fuel JP-10.[5]
α-Pinene is shown to have anti-inflammatory and memory-boosting properties.[8]
References
Bibliography
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads