Top Qs
Timeline
Chat
Perspective
Plutonium nitride
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Plutonium nitride is a binary inorganic compound of plutonium and nitrogen with the chemical formula PuN.[1][2]
Remove ads
Synthesis
Plutonium nitride can be prepared by the reaction of plutonium hydrides with nitrogen or ammonia at a temperature of 650 °C and a pressure of 0.3 kPa.[3]
Another method to prepare plutonium nitride is from the reduction of plutonium(III) iodide with sodium in liquid ammonia:
- PuI3 + NH3 + 3Na → PuN + 3NaI + 3/2H2
Other reactions can also produce plutonium nitride:[4]
- Pu + 1/2N2 + 3/2H2 → PuN + 3/2H2 (at 1000°C)
- PuCl3 + 1/2N2 + 3/2H2 → PuN + 3HCl (at 800–900°C)
Remove ads
Physical properties
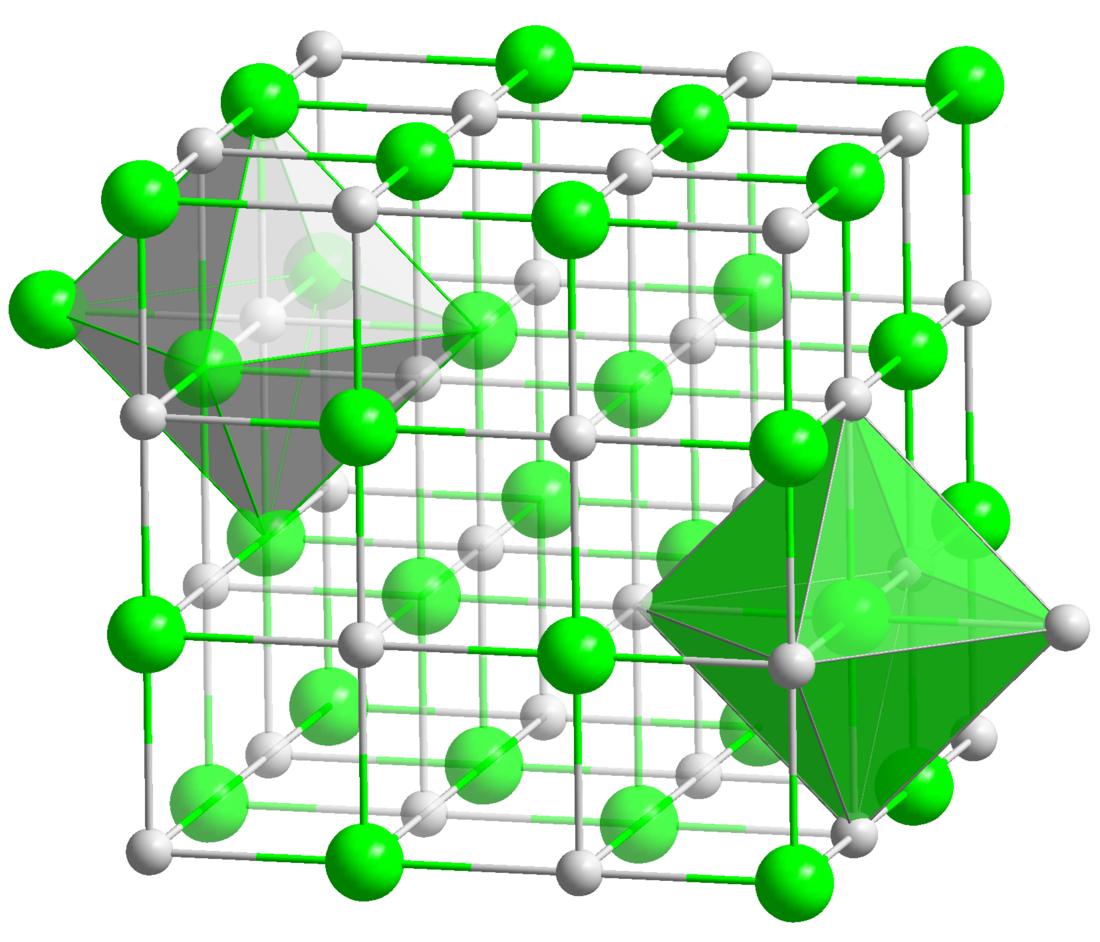
Plutonium nitride forms grey crystals in the cubic system with Fm3m space group.[5][6] It can be dissolved electrochemically.[7]
Uses
Plutonium nitride can be used as fuel in nuclear reactors.[8][9]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads