Top Qs
Timeline
Chat
Perspective
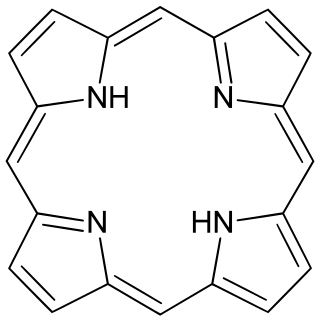
Porphine
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Porphine or porphin is an organic compound of empirical formula C20H14N4. It is heterocyclic and aromatic. The molecule is a flat macrocycle, consisting of four pyrrole-like rings joined by four methine bridges, which makes it the simplest of the tetrapyrroles.[2]
The nonpolar tetrapyrrolic ring structure of porphine means it is poorly soluble in most organic solvents and hardly water soluble.[3] As a result, porphine is mostly of theoretical interest. It has been detected in GC-MS of certain fractions of Piper betle.[4]
Remove ads
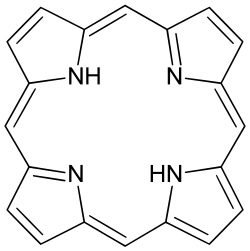
Porphine derivatives: porphyrins
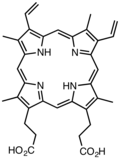
Substituted derivatives of porphine are called porphyrins. Many porphyrins are found in nature with the dominant example being protoporphyrin IX.[5] Many synthetic porphyrins are also known, including octaethylporphyrin[6] and tetraphenylporphyrin.[7]
- Common porphyrins
- Derivatives of protoporphyrin IX are common in nature, the precursor to hemes.
- Octaethylporphyrin (H2OEP) is a synthetic analogue of protoporphyrin IX. Unlike the natural porphyrin ligands, OEP2− is highly symmetrical.
- Tetraphenylporphyrin (H2TPP)is another synthetic analogue of protoporphyrin IX. Unlike the natural porphyrin ligands, TPP2− is highly symmetrical. Another difference is that its methine centers are occupied by phenyl groups.

Remove ads
Further reading
- Budavari, Susan (1989). "7574. Porphine". The Merck Index (11th ed.). Merck & Co., Inc. p. 1210. ISBN 0-911910-28-X. LCCN 89-60001.
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads