Top Qs
Timeline
Chat
Perspective
Potassium amide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Potassium amide is an inorganic compound with the chemical formula KNH2. Like other alkali metal amides, it is a white solid that hydrolyzes readily. It is a strong base.[1]
Remove ads
Production
Potassium amide is produced by the reaction of ammonia with potassium. The reaction typically requires a catalyst.[2]
Structure
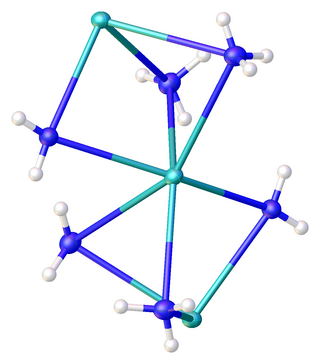
Traditionally KNH2 is viewed as a simple salt, but it has significant covalent character and is highly aggregated in ammonia solution.[citation needed] The compound has been characterized by X-ray crystallography as the solvent-free form[3] as well as the mono- and diammonia solvates. In KNH2·2NH3, the potassium centers are each bonded to two amido ligands and four ammonia ligands, all six of which bridge to adjacent potassium centers. The result is a chain of hexacoordinate potassium ions. The K–NH2 distances are 2.7652(11) whereas the K–NH3 distances are respectively 2.9234(11) and 3.0698(11) Å.[4]
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads