Top Qs
Timeline
Chat
Perspective
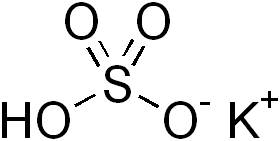
Potassium bisulfate
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Potassium bisulfate (potassium bisulphate) is an inorganic compound with the chemical formula KHSO4 and is the potassium acid salt of sulfuric acid. It is a white, water-soluble solid.
Remove ads
Preparation
More than 1 million tons were produced in 1985 as the initial stage in the Mannheim process for producing potassium sulfate. The relevant conversion is the exothermic reaction of potassium chloride and sulfuric acid:[1][2]
- KCl + H2SO4 → HCl + KHSO4
Potassium bisulfate is a by-product in the production of nitric acid from potassium nitrate and sulfuric acid:[3]
- KNO3 + H2SO4 → KHSO4 + HNO3
Chemical properties
Thermal decomposition of potassium bisulfate forms potassium pyrosulfate:[1]
- 2 KHSO4 → K2S2O7 + H2O
Above 600 °C potassium pyrosulfate converts to potassium sulfate and sulfur trioxide:[4]
- K2S2O7 → K2SO4 + SO3
Uses
Potassium bisulfate is commonly used to prepare potassium bitartrate for winemaking.[5] Potassium bisulfate is also used as a disintegrating agent in analytical chemistry or as a precursor to prepare potassium persulfate, a powerful oxidizing agent.[6]
Occurrence
Mercallite, the mineralogical form of potassium bisulfate, occurs very rarely.[7] Misenite is another more complex form of potassium bisulfate with the formula K8H6(SO4)7.
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads