Top Qs
Timeline
Chat
Perspective
Potassium hexachloroplatinate
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
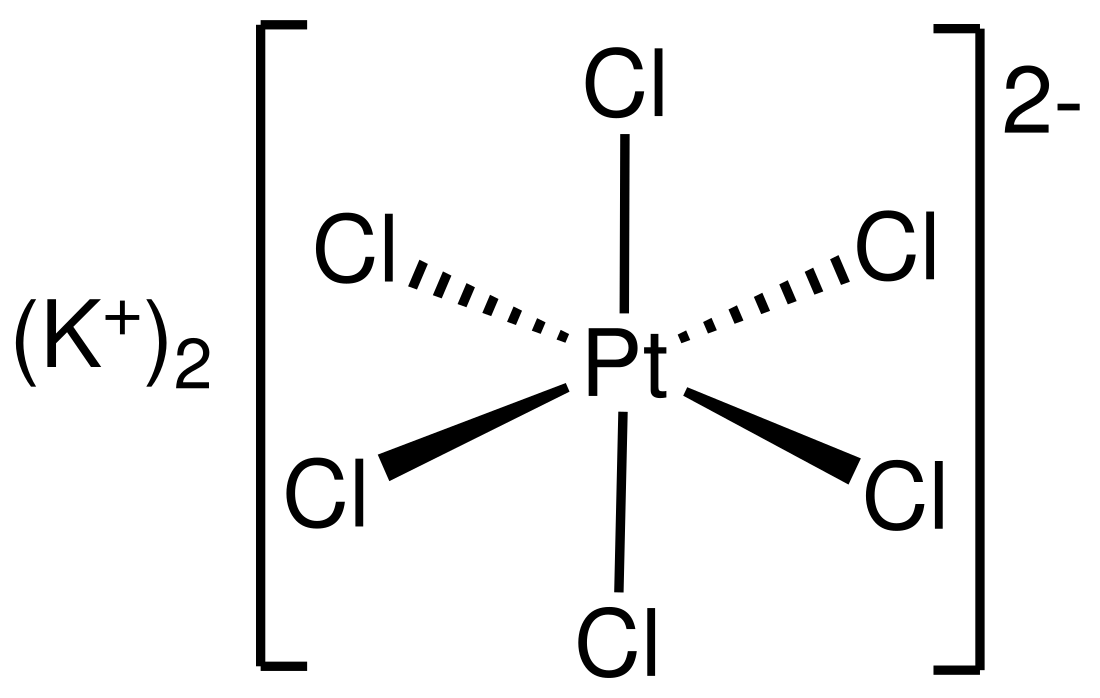
Potassium hexachloroplatinate is the inorganic compound with the formula K2PtCl6. It is a yellow solid that is a comparatively insoluble potassium salt. The salt features the hexachloroplatinate(IV) dianion, which has octahedral coordination geometry.
The precipitation of this compound from solutions of hexachloroplatinic acid was formerly used for the determination of potassium by gravimetric analysis.[4] It is also useful as an intermediate in the recovery of platinum from wastes.[5]
Remove ads
Reactions
Using salt metathesis reactions, potassium hexachloroplatinate is converted to a variety of quaternary ammonium and related lipophilic salts. These include tetrabutylammonium salt (NBu4)2PtCl6, which has been investigated as a catalyst.[6]
Reduction of potassium hexachloroplatinate with hydrazine dihydrochloride gives the corresponding tetrachloroplatinate salt.[7][8]
Potassium hexachloroplatinate reacts with aqueous ammonia to give chloropentammineplatinum chloride:[9]
- K2PtCl6 + 5 NH3 → [PtCl(NH3)5]Cl3 + 2 KCl
Remove ads
Safety
Dust containing potassium hexachloroplatinate can be highly allergenic. "Symptoms range from irritation of skin and mucous membranes to life-threatening attacks of asthma."[10]
Related compounds
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads