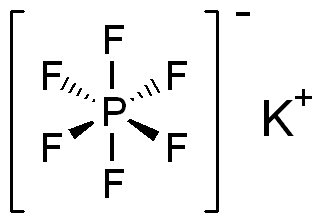Top Qs
Timeline
Chat
Perspective
Potassium hexafluorophosphate
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Potassium hexafluorophosphate is a chemical compound with the formula KPF6. This colourless salt consists of potassium cations and hexafluorophosphate anions. It is prepared from phosphorus pentachloride:[2]
- PCl5 + KCl + 6 HF → KPF6 + 6 HCl
This exothermic reaction is conducted in liquid hydrogen fluoride. The salt is stable in a hot alkaline aqueous solution, from which it can be recrystallized. The sodium and ammonium salts are more soluble in water whereas the rubidium and caesium salts are less so.
KPF6 is a common laboratory source of the hexafluorophosphate anion, a non-coordinating anion that confers lipophilicity to its salts. These salts are often less soluble than the closely related tetrafluoroborates.
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads